赵波庆1,2,李 娜1,2,陈 琛1,2,史肃龙1,2,班延鹏1,2,宋银敏1,2,何润霞1,2,刘全生1,2
(1.内蒙古工业大学 化工学院,内蒙古 呼和浩特 010051; 2.内蒙古工业大学 内蒙古自治区低阶碳质资源高值功能化利用重点实验室,内蒙古 呼和浩特 010051)
摘 要:褐煤的热解反应是褐煤利用的重要研究方向之一。为了分析褐煤热解过程中结构演变及气体生成机理,首先将胜利褐煤(SL)在固定床上进行热解制焦,利用800 ℃时SL热解气体生成速率曲线选取半焦终温,同时用气相色谱在线检测其所生成的热解气;其次结合煤焦傅里叶变换红外光谱(FT-IR)的表征进行分析,将半焦的FT-IR分峰拟合计算;最后将计算参数结合热解气生成规律,提出了热解升温过程中各反应阶段生成气体机理和气体生成过程中煤体结构的演变规律。结果表明,SL具有羟基、脂肪烃、芳环、羰基、醚键等丰富的官能团,热解温度低于350 ℃,胜利褐煤中主要官能团未发生明显变化;350~450 ℃,脂肪族侧链含氧官能团分解,热解温度450 ℃比350 ℃时煤焦中C![]() O相对含量(C1)降低78%;560~800 ℃,热解反应主要以芳香烷基侧链含氧官能团裂解为主,热解温度800 ℃时煤焦中C—O相对含量(C2)比560 ℃时降低27%;热解温度710~800 ℃时,煤热解以缩聚反应为主,热解温度800 ℃煤焦中芳香稠和度(D2)比710 ℃时升高65%。对4种热解气生成过程进行研究分析,CO2主要来源于中低温区煤中不同结构的羧基官能团分解;高温区生成CO,来源于煤中酚类、醚类、含氧杂环等结构的分解;CH4主要由芳环侧链的甲基、亚甲基或连接芳环结构亚甲基的分解;高温区产生的约60%H2主要来自于煤中芳香结构的缩聚反应。
O相对含量(C1)降低78%;560~800 ℃,热解反应主要以芳香烷基侧链含氧官能团裂解为主,热解温度800 ℃时煤焦中C—O相对含量(C2)比560 ℃时降低27%;热解温度710~800 ℃时,煤热解以缩聚反应为主,热解温度800 ℃煤焦中芳香稠和度(D2)比710 ℃时升高65%。对4种热解气生成过程进行研究分析,CO2主要来源于中低温区煤中不同结构的羧基官能团分解;高温区生成CO,来源于煤中酚类、醚类、含氧杂环等结构的分解;CH4主要由芳环侧链的甲基、亚甲基或连接芳环结构亚甲基的分解;高温区产生的约60%H2主要来自于煤中芳香结构的缩聚反应。
关键词:褐煤;热解;结构演变;热解气;生成机理
中图分类号:TQ530.2
文献标志码:A
文章编号:0253-9993(2019)02-0596-08
收稿日期:2018-04-17
修回日期:2018-10-16
责任编辑:常明然
基金项目:国家自然科学基金资助项目(21868021,21676149);内蒙古自治区自然科学基金资助项目(2018BS02007)
作者简介:赵波庆(1993—),男,山东日照人,硕士研究生。 E-mail:975225174@qq.com
通讯作者:李 娜(1987—),女,内蒙古赤峰人,讲师。E-mail:nali87@imut.edu.cn
赵波庆,李娜,陈琛,等.胜利褐煤热解过程中结构演变及气体生成机理分析[J].煤炭学报,2019,44(2):596-603.doi:10.13225/j.cnki.jccs.2018.0514
ZHAO Boqing,LI Na,CHEN Chen,et al.Analysis of structural evolution and gas generation mechanism during the pyrolysis of Shengli lignite[J].Journal of China Coal Society,2019,44(2):596-603.doi:10.13225/j.cnki.jccs.2018.0514
ZHAO Boqing1,2,LI Na1,2,CHEN Chen1,2,SHI Sulong1,2,BAN Yanpeng1,2,
SONG Yinmin1,2,HE Runxia1,2,LIU Quansheng1,2
(1.College of Chemical Engineering,Inner mongolia University of Technolegy,Huhhot 010051,China; 2.Inner Mongolia Key Laboratory of High-Value Funtional Utilization of Low Rank Carbon Resources,Inner mongolia University of Technolegy,Huhhot 010051,China)
Abstract:Pyrolysis reaction of lignite is one of the important research directions in lignite utilization.Firstly,Shengli Lignite (SL) was pyrolyzed on a fixed bed for coking in order to analyze the structure evolution and gas formation mechanism of lignite during pyrolysis.The final temperature of semi-coke was determined by using the curve of SL pyrolysis gas generation rate at 800 ℃.The pyrolytic gases was detected on-line by gas chromatography.Secondly,the characterization of Fourier transform infrared spectroscopy (FT-IR) of coal char is analyzed.And the FT-IR peak of semi-coke is fitted and calculated.Finally,combining the calculation parameters with the law of pyrolysis gas generation,the mechanisms of gas generation in each reaction stage during pyrolysis heating and the evolution law of coal structure in the process of gas generation were proposed.The results show that SL has abundant functional groups such as hydroxyl,aliphatic hydrocarbon,aromatic ring,carbonyl and ether bond.And the main functional groups in lignite have not changed,when the pyrolysis is below 350 ℃.Aliphatic oxygen-containing functional groups are decomposed between 350 and 450 ℃.The C![]() O relative content (C1) at the pyrolysis final temperature of 450 ℃ is 78% lower than that of 350 ℃.When the pyrolysis temperature is between 560 and 800 ℃,the pyrolysis reaction is mainly based on the decomposition of oxygen-containing functional groups of aromatic alkyl side chains.And the C—O relative content (C2) at the pyrolysis final temperature of 800 ℃ is 27% lower than that of 450 ℃.At 710-800 ℃ range,the main pyrolysis reaction is polycondensation.The aromatic condensation (D2) of chars at a final pyrolysis temperature of 800 ℃ is 65% higher than that at 710 ℃.Four kinds of pyrolysis gas generation processes were studied and analyzed that CO2 is mainly derived from the decomposition of carboxyl functional groups of different structures in the middle and low temperature zone.CO is generated in the high temperature,which is derived from the decomposition of the structures of phenol,ether and oxygen containing heterocyclic rings in coal.The decomposition of CH4 is mainly from the aromatic ring or methylene connecting the aromatic rings.The approximate 60% H2 mainly comes from the polycondensation reaction of aromatic structures in coal at a high-temperature zone.
O relative content (C1) at the pyrolysis final temperature of 450 ℃ is 78% lower than that of 350 ℃.When the pyrolysis temperature is between 560 and 800 ℃,the pyrolysis reaction is mainly based on the decomposition of oxygen-containing functional groups of aromatic alkyl side chains.And the C—O relative content (C2) at the pyrolysis final temperature of 800 ℃ is 27% lower than that of 450 ℃.At 710-800 ℃ range,the main pyrolysis reaction is polycondensation.The aromatic condensation (D2) of chars at a final pyrolysis temperature of 800 ℃ is 65% higher than that at 710 ℃.Four kinds of pyrolysis gas generation processes were studied and analyzed that CO2 is mainly derived from the decomposition of carboxyl functional groups of different structures in the middle and low temperature zone.CO is generated in the high temperature,which is derived from the decomposition of the structures of phenol,ether and oxygen containing heterocyclic rings in coal.The decomposition of CH4 is mainly from the aromatic ring or methylene connecting the aromatic rings.The approximate 60% H2 mainly comes from the polycondensation reaction of aromatic structures in coal at a high-temperature zone.
Key words:lignite;pyrolysis;structural evolution;pyrolysis gases;generation mechanism
随着全球变暖及环境污染等问题的日渐凸显,传统能源的清洁、高效利用已经成为亟待解决的问题[1]。褐煤因其固定碳及发热量低、挥发分及灰分高的特点,直接利用能效低且造成严重环境污染[2]。传统的利用方式(煤的气化、燃烧、液化等反应过程)都伴随着热解[3-4]。煤的热解反应没有其它反应物参与,仅为煤自身的热演变历程,故对煤热解过程的研究更能体现煤在整个热反应过程的本征信息。同时,褐煤提质炼焦是解决褐煤品质,提高其热稳定性的有效手段之一[5]。煤焦的形态结构与其反应特性密切相关,因此研究褐煤热解过程气相产物的形成及其产生机理是褐煤清洁利用的理论基础[6]。
煤作为一种复杂大分子有机复合体,在热解反应过程中既有大分子裂解反应,也存在分子间的缩合反应。煤自身结构的复杂性与反应的多样性使学者一直对煤的热解过程及机理进行不断的探究[7-8]。刘钦甫等[9]利用TG-IR-MS探究不同煤阶热解及氮的释放行为,发现不同煤化程度的煤会以不同的氮形式释放出来。在研究褐煤热解固相产物中,YE等[10]通过在不同升温速率下考察了热解过程中氧的释放规律,认为升温速率是影响氧转移的主要影响步骤。邹晓鹏等[11]利用XRD技术分析煤的碳微晶结构,发现随着煤化程度增加,碳结构有序化程度提高,认为这可能是造成煤样反应活性降低的重要原因。除了对煤样在热解过程中部分结构的演变进行分析,一些学者[12]在褐煤热解气态产物分布规律方面也进行了系统的研究。笔者所在课题组前期研究发现[13],胜利褐煤热解过程中固有矿物质对热解气体逸出顺序影响不大,但对CO2,CO和CH4生成均有抑制作用,对H2生成有促进作用。目前,将热解气体逸出规律与固相产物变化相结合进行研究较少。而热解气的逸出必然影响固相产物的结构形态发生变化,固相在热解过程中的化学反应也决定了热解气的生成,两者相互影响,将两者进行比对研究,更能具体而清晰的解析褐煤热解反应历程。
在课题组前期研究基础上,选用内蒙古锡林郭勒盟的胜利褐煤,着重探讨了胜利褐煤在热解过程气体逸出规律,并利用FT-IR技术对不同终温下得到的煤焦样品结构进行详细分析,阐述了热解过程结构演变与气体生成机理,为内蒙古褐煤的清洁利用提供了理论依据。
选用锡林浩特市胜利煤田2号矿褐煤为实验煤样,其中收到基水分(Mar)31.89%、灰分(Aar)7.04%、挥发分(Var)25.56%及固定碳(FCar)35.51%。煤样经破碎、筛分选取粒径为2~4 mm,在105 ℃下干燥4 h为实验用煤。
实验采用自制固定床热解反应装置对胜利褐煤进行热解提质,其反应装置流程如图1所示。
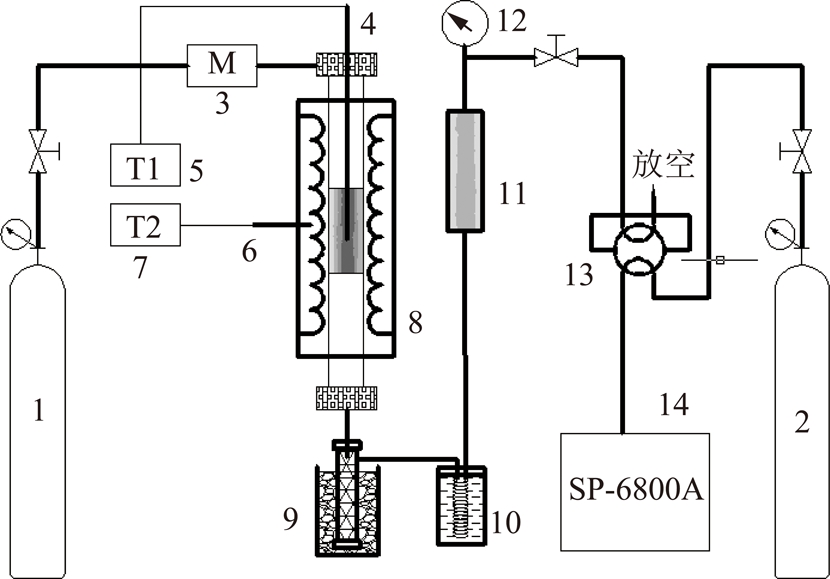
图1 热解反应器示意
Fig.1 Schematic representation of pyrolysis reactor
1—反应载气;2—色谱载气;3—质量流量计;4—测温热电偶;5—测温表;6—控温热电偶;7—控温表;8—反应器;9—冰浴;10—冷井;11—净化器;12—背压表;13—六通阀;14—气相色谱
反应器中胜利褐煤煤样的装填量为15±0.01 g,控制系统压力为0.2 MPa。首先,以20 ℃/min升温速率由室温升到105 ℃,恒温1 h,再以10 ℃/min升温速率将煤样分别加热到热解终温(350,450,560,710,800 ℃),并恒温2 h,升温过程载气为氩气,流速为500 mL/min。不同热解温度所制半焦样品分别记为350J,450J,560J,710J,800J。热解气体经过冷凝净化后进入气相色谱在线定性定量检测。热解气体生成速率及累积收率为
(1)
![]() (2)
(2)
FT,i,j=![]() Fi,jdt(3)
Fi,jdt(3)
式中,i为检测气体H2,CO,CH4和CO2;yi,out为气体i的体积浓度,%;V为载气Ar流量,500 mL/min;Vtotal为反应后干基出口气体总流量,mL/min;Fi为气体i的生成速率;FT,i,j为j ℃时H2,CO,CH4及CO2的累积收率,mL/g。
收集半焦称重后在干燥器中保存。煤样的热解产物产率根据称重质量差减法计算得到,半焦收率Ychar,焦油收率Ytar和气体质量收率Ygas为
(4)
![]() (5)
(5)
Ygas=100%-Ychar-Ytar-Mar(6)
式中,m0,mchar和mtar分别为煤样的初始质量,焦样质量和焦油质量。
实验采用美国尼高力公司NEXUS670型FT-IR红外光谱仪。实验样品与KBr以1∶400进行混合压片,在红外分析仪于400~4 000 cm-1范围采集红外谱图,分辨率为4 cm-1。
图2为热解终温为800 ℃时胜利褐煤煤样热解气体生成速率曲线。由图2可知,热解气体逸出顺序为CO2,CH4,CO和H2。最大生成速率对应温度分别为CO2:450 ℃;CH4:560 ℃;CO和H2为710 ℃。因此认为在该温度点,热解半焦结构发生较大转变,选取350,450,560,710与800 ℃为后续实验热解终温,分析热解气体与热解半焦的结构特点。
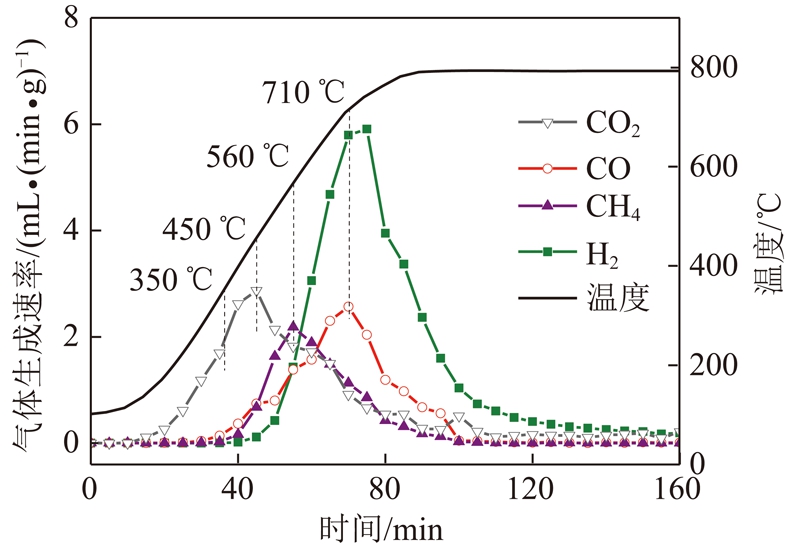
图2 胜利褐煤热解气体生成速率曲线
Fig.2 Released rates of pyrolysis gas productions of Shengli lignite
煤焦、热解气及焦油的收率及煤焦工业分析、元素分析结果见表1。随着热解温度的提高,煤焦收率逐渐下降,当热解终温达710 ℃,提高热解温度,煤焦收率变化不大;热解气产率随着热解温度的提高而升高;焦油产率在热解终温为450 ℃时最大,热解温度继续升高,焦油产率反而下降。
煤焦样品固定碳含量随着热解温度提高而提高;挥发分含量随着热解温度增高而降低;水、灰含量在热解过程中变化不大。碳元素含量随热解终温提高显著提高;氢与氧元素含量都有所下降,氧含量在热解终温达710 ℃显著降低,由此可看出,胜利褐煤在整个热解过程中结构组成发生了较大的变化。
图3为胜利褐煤煤样及半焦的红外光谱图。由图3可知,胜利褐煤和350J中含有强的羟基伸缩振动峰(3 409 cm-1)、甲基和亚甲基的C—H伸缩振动峰(2 921和2 856 cm-1)、羰基化合物的C![]() O伸缩振动峰(1 700 cm-1)、苯骨架C—C振动峰(1 600 cm-1附近)以及醚键—O—的伸缩振动振动峰(1 438 cm-1)[14-16]。比较胜利褐煤与350J红外
O伸缩振动峰(1 700 cm-1)、苯骨架C—C振动峰(1 600 cm-1附近)以及醚键—O—的伸缩振动振动峰(1 438 cm-1)[14-16]。比较胜利褐煤与350J红外
表1 半焦热解产物产率、工业分析及元素分析
Table 1 Production rate, industrial and elemental analysis of semi-coke pyrolysis products%

注:ad/d/daf为空干基/干基/干燥无灰基;A为灰分;V为挥发分;FC为固定碳;O*为差减得到。

图3 胜利褐煤与半焦红外谱图
Fig.3 FT-IR spectrogram of Shengli lignite and chars
谱图,发现其谱峰位置与峰强度均未有明显变化,表明热解终温在350 ℃前,胜利褐煤中的羟基、羰基、芳香族和脂肪族都未发生较大的变化。热解终温在450 ℃后,煤中的羟基振动峰、芳香氢和脂肪氢的C—H振动峰、亚甲基C—H振动峰强度减弱,羰基振动峰强度也明显降低,说明当热解温度达到450 ℃时,连接苯环的羟基、烷基(甲基、亚甲基和次甲基)和羰基断开。随着热解温度升高至560 ℃,3 100 cm-1处的芳香氢和2 800~3 000 cm-1处脂肪氢的C—H振动峰强度也逐渐减弱并在800J红外谱图中基本消失。苯骨架C—C振动峰随着热解终温的升高逐渐降低,在710J中振动峰强度最低,而随着热解终温进一步提升至800 ℃,在1 520~1 450 cm-1及900~700 cm-1处苯环上烯烃取代基的面外伸缩振动峰强度也有所增强。
图4为胜利褐煤拟合示意,对红外图谱进行官能团的定量计算[17],将红外光谱分为羟基伸缩振动和缔合峰(3 750~3 000 cm-1)、脂肪氢伸缩振动峰(3 000~2 800 cm-1)、苯环与含氧官能团伸缩振动峰(1 800~1 000 cm-1)、芳香氢弯曲振动峰(900~650 cm-1)4段进行分峰,拟合拟合结果见表2[14,18-20],拟合参数R2>0.99。
红外谱图参数计算[15,21]如下:
(1)R1~3可分别表示煤中脂肪氢、芳香氢及芳香度的变化规律。R1和R2值降低表示煤中脂肪氢芳香氢含量降低。R3值表示脂肪氢与芳香氢的比值,R3降低煤的芳香度升高。A(脂肪氢)=A1+A2+A3+A4,A(芳香氢)=A19+A20+A21+A22+A23+A24,Atotal为4段拟合的总面积之和。
R1=A(脂肪氢)/Atotal(7)
R2=A(芳香氢)/Atotal(8)
R3=A(芳香氢)/A(脂肪氢)(9)
图5为胜利褐煤和半焦FT-IR结构参数图。R1值在560J处比胜利褐煤降低45%,而在710J处升高至0.16,当热解终温为800 ℃时R1降低至0.09;R2值趋势与R1相反,560J较胜利褐煤增加71%;R3值的趋势与R1值相同,随热解终温升高煤体芳香度降低,但710J处R3值较560J升高,这可能是由于芳香结构裂解造成的[22]。
(2)C1和C2表示煤中C![]() O和C—O的相对含量变化规律。C3为煤的煤变质程度,C3值越低变质程度越高,煤阶越高。A(C
O和C—O的相对含量变化规律。C3为煤的煤变质程度,C3值越低变质程度越高,煤阶越高。A(C![]() O)=A10,A(C—O)=A15+A16。
O)=A10,A(C—O)=A15+A16。
C1=A(C![]() O)/Atotal(10)
O)/Atotal(10)
C2=A(C—O)/Atotal(11)
C3=A(C![]() O)/(A(C
O)/(A(C![]() O)+A(C—O))(12)
O)+A(C—O))(12)
煤中含氧官能团的变化规律由图6可知。随着热解终温升高中C1值不断降低,C1值在350J比450J减少78%,C1值在热解终温为560 ℃以上几乎为0,说明中C![]() O的分解主要在中低温区发生;而C2值热解终温350~560 ℃过程中相对稳定,560J到800J降低27%,这很有可能说明多数醚键和C—O在中高温区分解[23];C3值在350J是450J的5.3倍,在此温区煤变质程度明显升高。
O的分解主要在中低温区发生;而C2值热解终温350~560 ℃过程中相对稳定,560J到800J降低27%,这很有可能说明多数醚键和C—O在中高温区分解[23];C3值在350J是450J的5.3倍,在此温区煤变质程度明显升高。
(3)D1表示煤芳香结构的相对含量变化规律,D2表示芳香结构稠和程度,D2值越高表示芳香稠和程度越高。A(芳环C![]() C)=A11。
C)=A11。
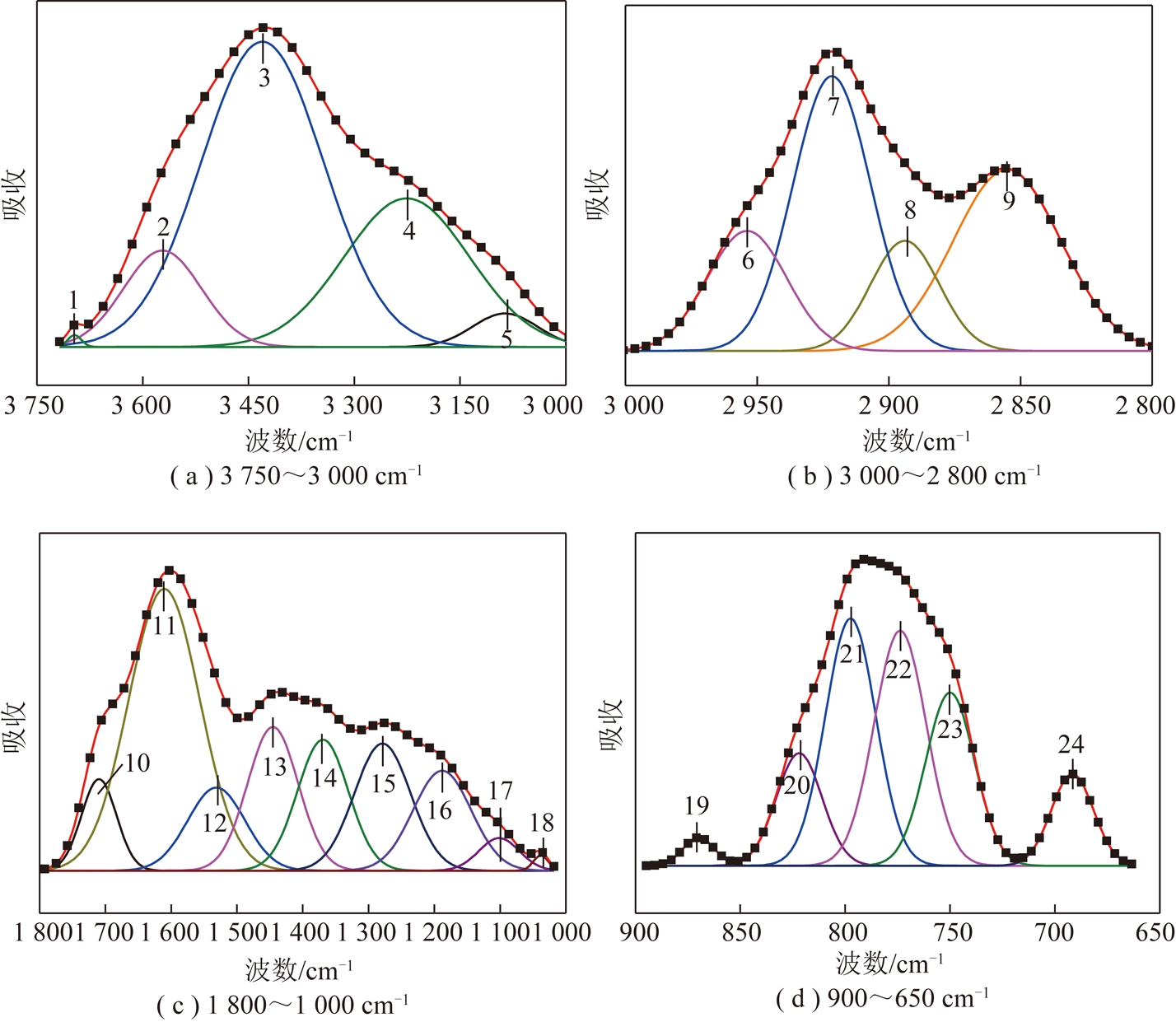
图4 不同波段胜利褐煤红外光谱拟合曲线
Fig.4 Curve fittings of Shengli lignite’s infrared spectrum in different wavenumber bands
表2 胜利褐煤拟合参数
Table 2 Parameters of fitted peaks from Shengli lignite sample’s infrared spectrum

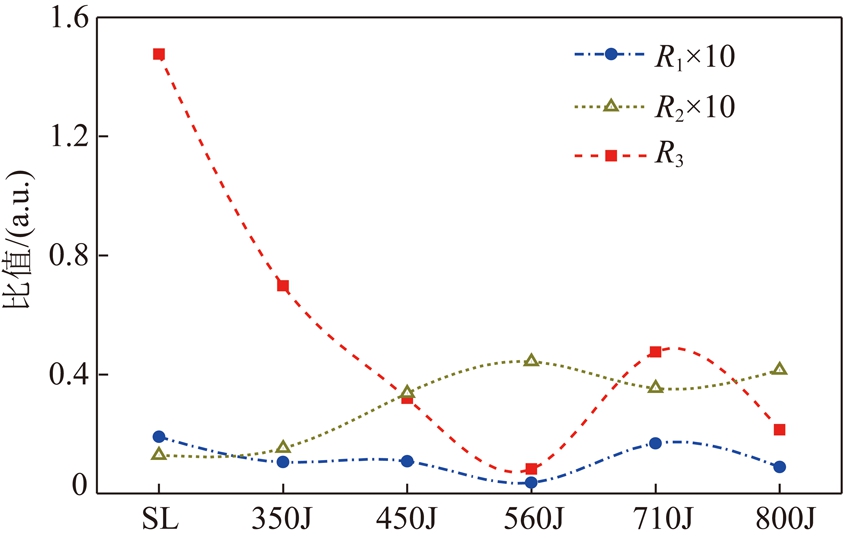
图5 胜利褐煤和半焦R1~3结构参数
Fig.5 R1~3 structural parameters of Shengli lignite and chars
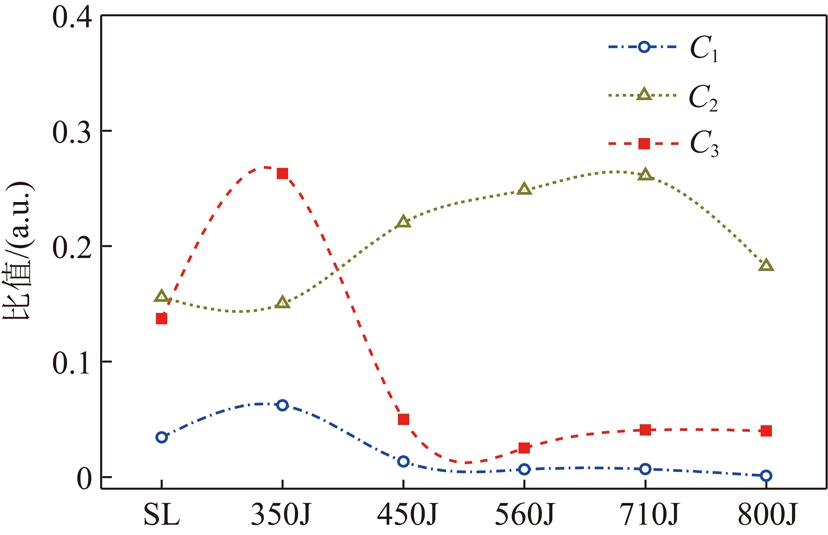
图6 胜利褐煤和半焦C1~3结构参数
Fig.6 C1~3 structural parameters of Shengli lignite and chars
D1=A(芳环C![]() C)/Atotal(13)
C)/Atotal(13)
D2=A(芳香氢)/A(芳环C![]() C)(14)
C)(14)
由图7热解过程中胜利褐煤芳香程度变化可知,D1值热解终温在450 ℃比800 ℃时降低19%,表明芳香结构减少;D2值随着热解终温增加而增加,其中热解终温在710 ℃比800 ℃时增加65%,表明芳香稠和程度增加。
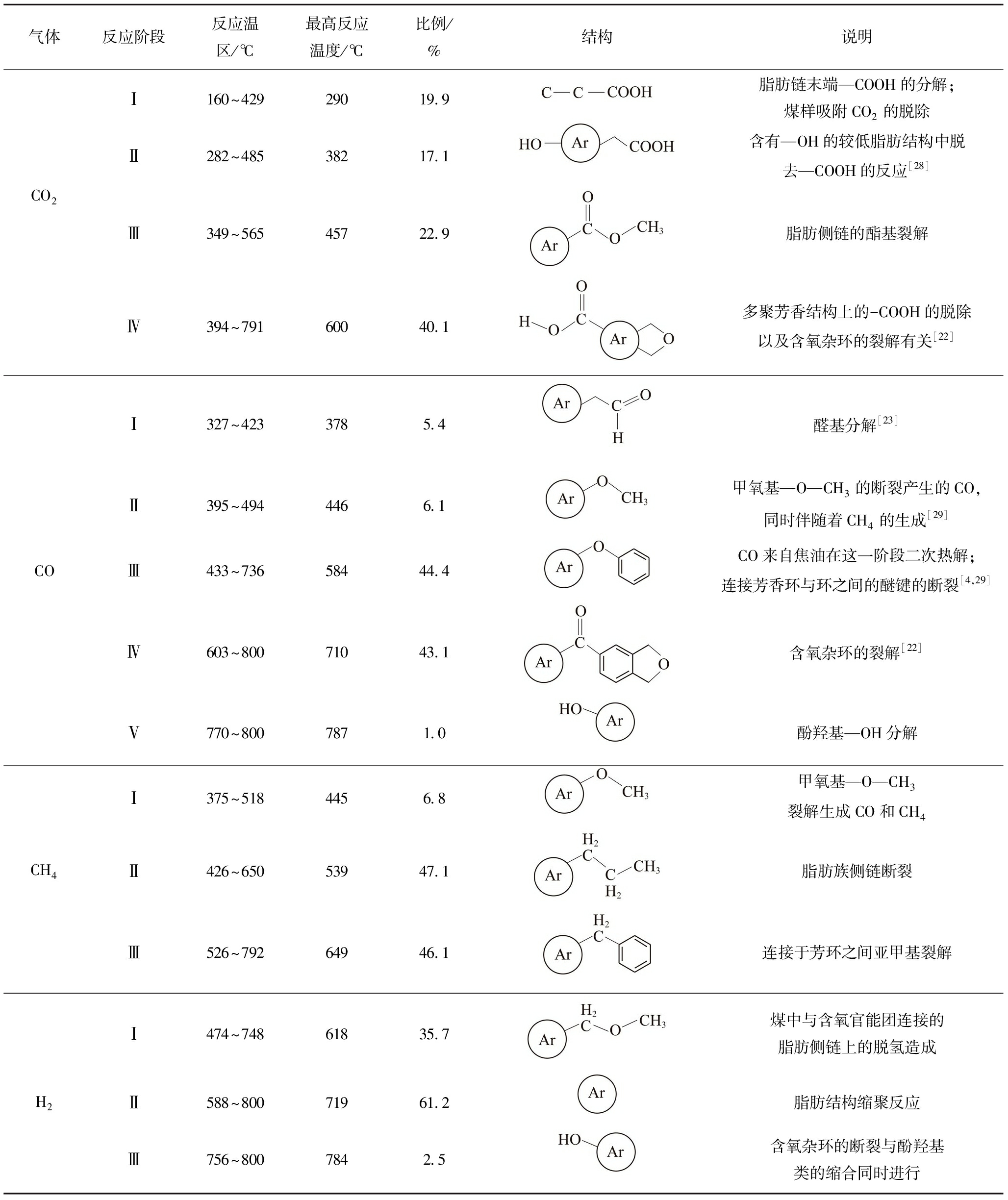
随着热解终温的升高,热解气体CO2,CO,CH4,H2的出峰规律发生变化,但同一气态产物在不同热解温区内是由不同结构产生的,对热解终温800 ℃煤样的气体生成速率曲线根据实验数据温区进行高斯分峰拟合[24-25]为图8,拟合相关度指数R2>0.99。对比半焦FT-IR分析得到的煤样结构变化,进行热解过程中气态产物机理的分析,褐煤气体生成机理如图8所示。由图8可知,CO2的生成主要发生在200~600 ℃,这是由煤中不同形式的羧基造成[26]。而CO生成主要在中高温区,有研究表明[27],CO的来源与煤中醚类、含氧杂环和酚类关系密切。CH4的生成机理相对简单,主要是煤中不同形式的甲基亚甲基的断裂[24]。H2的生成主要来源于高温区煤的缩聚反应。具体各反应阶段的机理分析由表3给出。
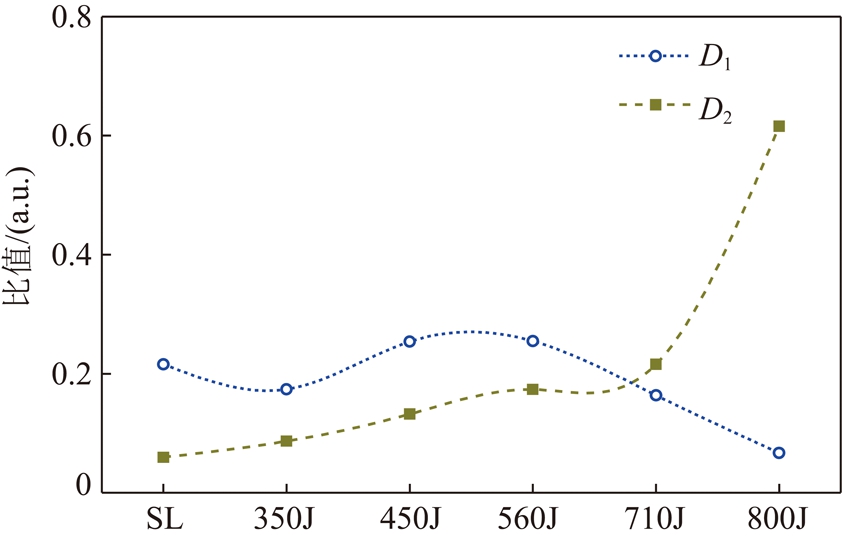
图7 胜利褐煤和半焦D1~2结构参数
Fig.7 D1~2 structural parameters of Shengli lignite and chars

图8 胜利褐煤热解气体生成机理
Fig.8 Mechanism for the gases generation during pyrolysis of Shengli lignite
(1)热解气CO2主要来自于600 ℃以前煤中不同形式羧基的分解,依次为脂肪侧链、芳香结构上的—COOH分解产生,煤变质程度C3值在350J是450J的5.3倍,在此温区煤变质程度明显升高。
(2)CO生成主要在中高温区,由醛基、甲氧基、芳环环间醚键和环内醚键分解产生,热解终温800 ℃煤中C—O相对含量(C2)比560 ℃时降低27%。
(3)CH4的生成主要由甲基亚甲基断裂产生,450~800 ℃温区产生的CH4占总产生甲烷气90%以上。
(4)H2产生多发生在高温区,约60%的H2由缩聚反应产生。
表3 各反应阶段热解生成气机理分析
Table 3 Mechanism analysis of pyrolysis gases at various reaction stages
参考文献
[1] SONG H J,LIU G R,WU J.Pyrolysis characteristics and kinetics of low rank coals by distributed activation energy model[J].Energy Conversion Management,2016,126:1037-1046.
[2] LIU P,ZHANG D X,WANG L L,et al.The structure and pyrolysis product distribution of lignite from different sedimentary environment[J].Applied Energy,2016,163:254-262.
[3] 刘源,贺新福,杨伏生,等.热解温度及气氛变化对神府煤热解产物分布的影响[J].煤炭学报,2015,40(2):497-502.
LIU Yuan,HE Xinfu,YANG Fusheng,et al.Impacts of pyrolysis temperature and atmosphere on product distribution of Shenfu coal pyrolysis[J].Journal of China Coal Society,2015,40(2):497-502.
[4] ZHENG M,LI X X,LIU J,et al.Pyrolysis of Liulin coal simulated by GPU-based reaxFF MD with cheminformatics analysis[J].Energy & Fuels,2014,28(1):522-534.
[5] LIU P,WANG L L,ZHOU Y,et al.Effect of hydrothermal treatment on the structure and pyrolysis product distribution of Xiaolongtan lignite[J].Fuel,2016,164:110-118.
[6] 宋昱,朱炎铭,李伍.东胜长焰煤热解含氧官能团结构演化13C-NMR和FT-IR分析[J].燃料化学学报,2015,43(5):519-528.
SONG Yu,ZHU Yanming,LI Wu.Structure evolution of oxygen functional groups in Dongsheng long flame coal by13C-NMR and FT-IR[J].Journal Fuel Chemistry Technology,2015,43(5):519-528.
[7] WIJAYA N,ZHANG L.A critical review of coal demineralization and its implication on understanding the speciation of organically bound metals and submicrometer mineral grains in coal[J].Energy & Fuels,2011,25(1):1-16.
[8] LI G Y,DING J X,ZHANG H,et al.ReaxFF simulations of hydrothermal treatment of lignite and its impact on chemical structures[J].Fuel,2015,154:243-251.
[9] 刘钦甫,徐占杰,崔晓南,等.不同煤化程度煤的热解及氮的释放行为[J].煤炭学报,2015,40(2):450-455.
LIU Qinfu,XU Zhanjie,CUI Xiaonan,et al.Release behavior of nitrogen in different rank coals during pyrolysis[J].Journal of China Coal Society,2015,40(2):450-455.
[10] YE C P,HUANG H J,LI X H,et al.The oxygen evolution during pyrolysis of HunlunBuir lignite under different heating modes[J].Fuel,2017,207:85-92.
[11] 邹晓鹏,盛羽静,陆海峰,等.粒径对不同煤阶煤焦气化反应活性的影响研究[J].燃料化学学报,2017,45(4):408-417.
ZOU Xiaopeng,SHENG Yujing,LU Haifeng,et al.Effect of particle size on gasification of char with different coal ranks[J].Journal Fuel Chemistry Technology,2017,45(4):408-417.
[12] JAYARAMAN K,GOKALP I.Thermogravimetric and evolved gas analyses of high ash Indian and Turkish coal pyrolysis and gasification[J].Journal of Thermal Analysis and Calorimetry,2015,121(2):919-927.
[13] 周晨亮,刘全生,李阳,等.固有矿物质对胜利褐煤热解气态产物生成及其动力学特性影响的实验研究[J].中国机电工程学报,2013,33(35):21-27.
ZHOU Chenliang,LIU Quansheng,LI Yang,et al.Effect of inherent minerals on the production of pyrolysis gases and the corresponding kinetics for Shengli lignite[J].Proceedings of the CSEE,2013,33(35):21-27.
[14] QIN Z H,CHEN H,YAN Y J,et al.FTIR quantitative analysis upon solubility of carbon disulfide/N-methyl-2-pyrrolidinone mixed solvent to coal petrographic constituents[J].Fuel Processing Technology,2015,133:14-19.
[15] ZHAO Y,QIU P H,CHEN G,et al.Selective enrichment of chemical structure during first grinding of Zhundong coal and its effect on pyrolysis reactivity[J].Fuel,2017,189:46-56.
[16] MALUBAZO N,WAGNER N J,BUNT J R D,et al.Structural analysis of chars generated from South African inertinite coals in a pipe-reactor combustion unit[J].Fuel Processing Technology,2011,92(4):743-749.
[17] IBARRA J V,MU OZ E,MOLINER R.FTIR study of the evolution of coal structure during the coalification process[J].Organic Geochemistry,1996,24(6/7):725-735.
OZ E,MOLINER R.FTIR study of the evolution of coal structure during the coalification process[J].Organic Geochemistry,1996,24(6/7):725-735.
[18] MIURA K,MAE K,LI W,et al.Estimation of hydrogen bond distribution in coal through the analysis of OH stretching bands in diffuse reflectance infrared spectrum measured by in-situ technique[J].Energy & Fuels,2001,15:599-610.
[19] DONG P W,CHEN G,ZENG X,et al.Evolution of inherent oxygen in solid fuels during pyrolysis[J].Energy & Fuels,2015,29(4):2268-2276.
[20] 迟铭书,王擎,李松阳,等.酸洗对桦甸油页岩矿物质以及有机结构的影响[J].燃料化学学报,2017,45(12):1424-1433.
CHI Mingshu,WANG Qing,LI Songyang,et al.Influence of demineralization on minerals and organic structure in Huadian oil shale[J].Journal of Fuel Chemistry Technology,2017,45(12):1424-1433.
[21] 李娜,刘全生,甄明,等.不同变质程度煤燃烧反应性及FTIR分析其热解过程结构变化[J].光谱学与光谱分析,2016,36(9):2760-2765.
LI Na,LIU Quansheng,ZHEN Ming,et al.Coal combution reactivity of different metamorphic degree and strucrure changes of FTIR analysis in pyrolysis process[J].Spectroscopy and Spectral Analysis,2016,36(9):2760-2765.
[22] MENG F R,YU J L,TAHMASEBI A,et al.Characteristics of chars from low-temperature pyrolysis of lignite[J].Energy & Fuels,2013,28(1):275-284.
[23] MENG F R,YU J L,TAHMASEBI A,et al.Characteristics of chars from low-temperature pyrolysis of lignite[J].Energy & Fuels,2013,28(1):275-284.
[24] LIU J X,JIANG X M,SHEN J,et al.Pyrolysis of superfine pulverized coal.Part 1.Mechanisms of methane formation[J].Energy Conversion and Management,2014,87:1027-1038.
[25] LIU J X,JIANG X M,SHEN J,et al.Pyrolysis of superfine pulverized coal.Part 2.Mechanisms of carbon monoxide formation[J].Energy Conversion and Management,2014,87:1039-1049.
[26] IBARRA V J,MOLINER R,BONET A J.FT-IR investigation on char formation during the early stages of coal pyrolysis[J].Fuel,1993,73(6):918-924.
[27] MR ZIKOV
ZIKOV J,SINDLER S,VEVERKA L,et al.Evolution of organic oxygen bonds during pyrolysis of coal[J].Fuel,1986,65:342-345.
J,SINDLER S,VEVERKA L,et al.Evolution of organic oxygen bonds during pyrolysis of coal[J].Fuel,1986,65:342-345.
[28] HEEK K V,HODEK W.Structure and pyrolysis behaviour of different coals and relevant model substances[J].Fuel,1994,73:886-896.
[29] HODEK W,KIRSCHSTEIN J,HEEK K V.Reactions of oxygen containing structures in coal pyrolysis[J].Fuel,1991,70:424-428.