赵 晗1,4,何 环2,3,王江泽1,4,谭凯丽1,4,赵 娜1,4,任恒星1,4
(1.山西晋城无烟煤矿业集团有限责任公司 煤与煤层气共采国家重点实验室,山西 晋城 048000; 2.中国矿业大学 化工学院,江苏 徐州 221116; 3.中国矿业大学 煤炭加工与高效洁净利用教育部重点实验室,江苏 徐州 221116; 4.易安蓝焰煤与煤层气共采技术有限责任公司,山西 晋城 048000)
摘 要:为研究微生物群落在褐煤生物产气过程中的作用以及产气前后煤样性质的变化。以内蒙古胜利褐煤为产气底物,寺河矿区煤层气井排出水中富集产气微生物为出发菌群,在实验室开展褐煤生物产气实验,采用Illumina高通量测序平台分析产气前后微生物群落变化,并利用气相色谱、扫描电子显微镜等手段对褐煤产甲烷量和产气前后煤样物化性质及其煤表面的菌体形貌进行分析。结果表明,内蒙胜利褐煤可以被所富集得到的菌群利用并产生甲烷,产气周期为49 d,期间累计产甲烷量为83.1 mL,净产甲烷率为7.84 mL/g煤。褐煤生物产气微生物样本中细菌群落多样性丰富,主要优势菌门为厚壁菌门(Firmicutes)、变形菌门(Proteobacteria)、WWE1、拟杆菌门(Bacteroidetes)、互养菌门(Synergistetes)和少量的脱铁杆菌门(Deferribacteres)。原始微生物群落结构多样性较高,经褐煤和基本培养基培养产气后,群落多样性降低。在微生物属水平上菌群结构变化较大,其中W22,Proteiniclasticum,VadinCA02,Tissierella_ soehngenia,Clostridium,Desulfovibrio等菌属在产气过程中发挥重要功能。内蒙胜利褐煤挥发分较高,富氢、富氧,煤表面结构松散有明显裂隙,有利于微生物附着降解,适宜进行生物产气试验。褐煤经微生物作用产气后,水分、灰分、挥发分均降低,固定碳百分比升高,H/C升高,S元素比例下降,利用扫描电镜观察到产气体系中煤表面附着大量短杆状和球状微生物,并存在类似微生物纳米导线的结构。
关键词:褐煤;生物产气;群落变化;高通量测序
中图分类号:P618
文献标志码:A
文章编号:0253-9993(2019)04-1224-08
收稿日期:2018![]() 05
05![]() 21
21
修回日期:2018![]() 09
09![]() 22
22
责任编辑:常明然
基金项目:山西省煤层气联合研究基金资助项目(2016012009);中央高校基本科研业务费专项资金资助项目(2017XKQY037)
作者简介:赵 晗(1988—),女,山西晋城人,工程师。E-mail:zhaohan_2011@163.com
通讯作者:何 环(1981—),男,湖南平江人,副教授,硕士生导师。E-mail:hehuan6819@cumt.edu.cn
ZHAO Han1,4,HE Huan2,3,WANG Jiangze1,4,TAN Kaili1,4,ZHAO Na1,4,REN Hengxing1,4
(1.State Key Laboratory of Coal and CBM Co-mining,Shanxi Jincheng Anthracite Mining Group Co.,Ltd.,Jincheng 048000,China; 2.School of Chemical Engineering and Technology,China University of Mining and Technology,Xuzhou 221116,China; 3.Key Laboratory of Coal Processing and Efficient and Clean Utilization of Ministry of Education,China University of Mining and Technology,Xuzhou 221116,China; 4.Yi’an Lanyan Coal and Coal-bed Methane Simultaneous Extraction Technology Co.,Ltd.,Jincheng 048000,China)
Abstract:In order to study the role of microbial community in lignite biogas production process and the change of coal sample properties before and after gas production,the lignite obtained from Shengli in Inner Mongolia was used as the substrate for gas production,and the enriched anaerobic flora in Sihe mining area was used as the starting bacterial community.In the laboratory scale,the lignite biogas production was conducted.The microbial community changes before and after gas production were analyzed by Illumina high-throughput sequencing platform.Gas chromatography,scanning electron microscopy,etc.were used to analyze the methane production of lignite and the physicochemical properties of coal samples before and after gas production and the morphology of the coal surface.The results showed that Inner Mongolia Shengli lignite could be utilized by the enriched flora and produce methane with a biogas production cycle of 49 days,and the cumulative methane production was 83.1 mL.The net methane production rate was 7.84 mL/g coal.The diversity of bacterial communities in the biomass biogenic samples of lignite was abundant.The main dominant bacteria at phylum level were Firmicutes,Proteobacteria,WWE1,Bacteroidetes,Synergistetes and Deferribacteres.The initial microbial flora had high diversity,however,the microbial diversity decreased after the biogas generation with lignite and basic medium.The microbial structure changed obviously at genus level,and the dominant bacteria W22,Proteiniclasticum,VadinCA02,Tissierella soehngenia,Clostridium and Desulfovibrio played important roles in the biogas generation.Inner Mongolia Shengli lignite had higher volatile matter,rich in hydrogen and oxygen,and the surface structure of coal was loose,with obvious cracks,which was beneficial to microbial adhesion and degradation,and was suitable for biogas production.After the biogas production,lignite’s volatile content,water and ash,the proportion of S and O elements decreased,however the percentage of fixed carbon and the H/C increased.The SEM results showed that a large number of short rod-like and spherical microbes absorbed onto the surface of coal,and also had similar microorganism nanowire structure.
Key words:lignite;biogenic gas;microbial flora succession;high-throughput sequencing
赵晗,何环,王江泽,等.内蒙胜利褐煤生物产气前后微生物群落变化[J].煤炭学报,2019,44(4):1224-1231.doi:10.13225/j.cnki.jccs.2018.0681
ZHAO Han,HE Huan,WANG Jiangze,et al.Variation of microbial community before and after biogas production with Shengli lignite in Inner Mongolia[J].Journal of China Coal Society,2019,44(4):1224-1231.doi:10.13225/j.cnki.jccs.2018.0681
煤的生物产气是由产甲烷相关功能菌厌氧代谢煤或煤层物质产生的以甲烷为主要成分的气体[1-2]。已有研究表明,褐煤中含有较多有机物质,所以容易被本源和外源微生物降解形成生物气[3-4]。我国拥有丰富的褐煤资源,主要分布在内蒙和云南等地,其中内蒙褐煤约占我国褐煤资源的70%,因此对内蒙褐煤资源开展生物产气研究对拓宽煤炭清洁利用途径具有重要意义。
目前国内外学者对煤生物产气环境中微生物群落结构研究表明,不同来源样品中微生物群落组成差异较大[5-6]。HE等[7]发现山东赵楼一处不产煤层气煤层水样品中细菌种类丰富而产甲烷古菌丰度较低。而DARIUSZ等[8]研究发现产甲烷菌Methanocorpusculum是伊利诺斯盆地环境中的主要菌属。也有研究人员报道日本近海的海底煤层中存在一类能够直接利用煤中苯甲氧基化合物形成甲烷的古菌Methermicoccus[9-10]。
研究过程中也发现同一个取样点的煤层和煤层水中微生物群落也存在较大差异[11]。DONALD等[12]研究发现粉河盆地煤层中的产甲烷菌主要有Methanobacterium等古菌,而地下水中主要为Methanocaldococcus等古菌。GUO等[13]发现鄂尔多斯盆地中煤层水、煤和岩石表面微生物分布存在差异,其中煤和岩石表面的细菌组成较接近,但是和煤层水中细菌组成差异却很大,古菌Methanolobus在水、煤和岩石表面分布均较多,而Methanobacterium却仅在煤中有分布。VICK等[14]报道产气初期Desulfuromonas,Campylobacter,Sulfurospirillum和Streptobacillus是吸附到煤表面的微生物,而游离体系中丰度最高的微生物却是产甲烷古菌。可见,不同来源的环境样品以及同一样品不同时间和空间的微生物群落组成均存在差异。
也有研究人员报道不同来源富集到的外源环境微生物样品也可以利用其他矿区开采煤进行生物产气[6-7]。晋城是我国重要的无烟煤产区,前期研究表明该地区寺河矿煤层气抽采排出水中微生物富集样品可以利用无烟煤进行生物产气[15],但是否也可以利用褐煤产气却不清楚。笔者利用寺河矿区煤层气抽采井排出水富集菌群作为产气微生物,内蒙胜利褐煤作为产气底物,在实验室进行褐煤微生物产气模拟实验,采用高通量测序方法分析了产气前后微生物菌群组成变化情况,并对产气前后煤的物化性质进行了分析。
煤样取自内蒙古神华北电胜利露天煤矿褐煤5号煤层,试验时将煤样破碎筛分至1~2 mm颗粒进行生物产气模拟试验。其中煤工业分析和元素分析按照国家标准GB/T 30732—2014和GB/T 31391—2015进行。
实验采用寺河矿区煤层气井产出水,采集水样后置于厌氧发酵罐内添加培养基和寺河矿开采新鲜无烟煤煤块进行富集培养,培养基制备方法同文献[15]。定期放出部分菌液,补充等量新鲜培养基,减少有害代谢产物对菌群产气能力的影响,待产气能力稳定后作为菌液待用。
选用500 mL厌氧瓶进行褐煤产气实验,设置试验组和对照组,每组3个平行,实验数据为平均值。对照组加入250 mL培养基和50 mL菌液;试验组,加入等量培养基、菌液和10 g煤样。实验时,在厌氧工作站(英国,DWS DG1000)中按设置分装好培养基、菌液和煤样,密封保证厌氧环境,35 ℃恒温培养。每7 d检测气体组分,并记录产气量,同时在实验初期、末期分别取样进行高通量测序,分析微生物群落结构。
产气过程中气体组分采用美国Angilent 7890 气相色谱仪分析,配Carbonplot色谱柱(60 m×320 μm×1.5 μm)和 TCD 检测器,气密针进样,进样量0.5 mL。色谱进样口温度150 ℃,柱温箱温度30 ℃,检测器温度200 ℃[15]。甲烷体积分数=甲烷实际出峰面积×甲烷标气中甲烷体积分数/标气中甲烷平均峰面积。为准确研究煤样产甲烷率,通过去除对照组产甲烷量计算净产甲烷率。净产甲烷率计算公式为
Q=(qncn-q0c0)/m
式中,qn为试验组产气量,mL;cn为试验组产甲烷体积分数,%;q0为对照组产气量,mL;c0为对照组产甲烷体积分数,%;m为实验所用煤量,g。
取10 mL培养液(包含煤粉)样品10 000 rpm离心收集沉淀,采用试剂盒(EZNA water DNA kit,OMEGA)提取沉淀样品中基因组DNA,通过1.5%琼脂糖凝胶电泳检测抽提基因组的完整性,利用 Qubit2.0 DNA 试剂盒检测基因组 DNA 浓度。利用特异性引物PCR扩增V3-V4可变区。PCR产物进行琼脂糖电泳,采用DNA胶回收试剂盒(RTP2201,中科瑞泰)对 PCR产物进行回收。回收的PCR产物通过Qubit2.0 DNA 检测试剂盒精确定量,并添加测序标签,采用赛哲生物的 Illumina测序平台完成对样品高通量测序。测序结果采用分析软件将序列按照序列相似性为97%的阈值进行OTUs(operational taxonomic units)操作分类单元聚类,此外对样品序列进行Alpha多样性统计分析,包括ACE,Chao1,OTUs和Shannon等。其中Alpha多样性是指一个特定区域或生态系统内的多样性,是反映丰富度和均匀性的综合指标,群落丰富度的指数主要包括Chao1指数和ACE指数,群落多样性的指数主要包括Shannon指数和Simpson指数。
褐煤生物产气实验后,用无菌超纯水浸泡洗涤3次,室温下充分干燥后,对残煤进行工业分析和元素分析。另取部分产气前后煤样品用于电镜(ZEISS EVO MA15)观察,原煤干燥后直接用离子溅射仪喷金(ETD-2000C)观察,残煤先使用2.5%(V/W)戊二醛在pH 7.2的0.1 mol/L磷酸钾缓冲液固定2.5 h,然后对样品进行乙醇逐级脱水[16]并在空气干燥24~48 h后,喷金观察煤表面微生物形貌特征。
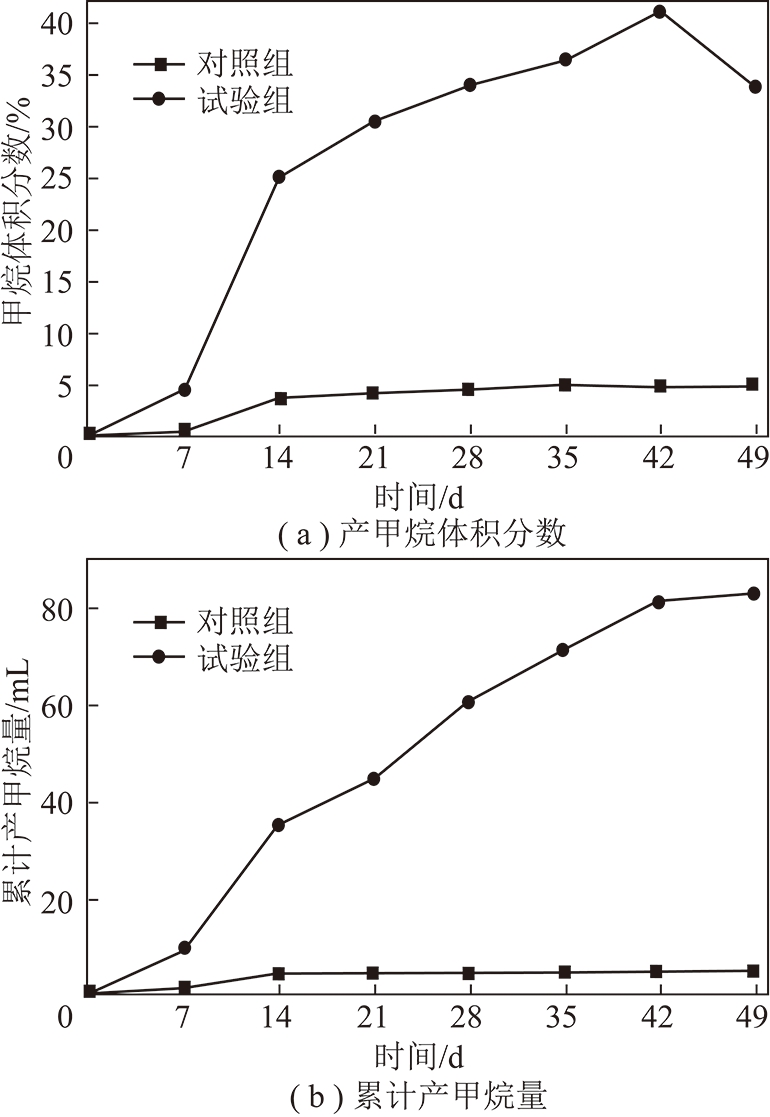
图1 褐煤产甲烷变化曲线
Fig.1 Methane product curves of lignite
褐煤生物产气试验结果如图1所示,对照组在0~7 d有短暂的延滞期,接种微生物利用培养基进行大量繁殖,7~14 d开始有甲烷产生,体积分数缓慢上升,在14 d达到峰值4%,利用完可用有机物后,产气停滞甲烷体积分数保持不变,累计产甲烷量为4.7 mL。相比之下试验组因反应体系中加有煤样,可利用底物丰富,微生物快速繁殖代谢,0~7 d甲烷体积分数达4.5%,7~14 d甲烷体积分数速增至25%,随后产气开始缓慢稳定的持续升高,峰值达41%,42 d后甲烷体积分数降低,产气周期结束。整个实验周期内累计产甲烷量为83.1 mL,净产甲烷率为7.84 mL/g。
有报道称褐煤利用外源产甲烷菌群的产气周期为28 d,可以分为产气速率显著增高、缓慢增高、趋于停止3个阶段[7],这与本研究中外源产甲烷菌群产气规律不同。原因可能有两方面:① 上述文献中所用煤样与本文不同,不同煤样具有不同的理化性质,可被微生物利用降解的程度也不相同;② 虽然同为产甲烷菌群,但菌群结构差异较大,文献所用菌源为驯化沼液,而本文为煤层水微生物富集培养得到。
表1为实验所用微生物在培养前后的Alpha多样性统计结果。其中寺河煤层水富集微生物样本中共观察到964个OTU,而经过产气培养后的对照组和试验组分别下降为368和436个OTU,结合Shannon值等多样性指数可以看出群落多样性由高到低分别为:原始>试验组>对照组,原始微生物群落结构多样性较高,经生物产气后,对照组与试验组群落结构发生较大变化。其中对照组只添加了少量培养基,营养单一,试验组添加了褐煤煤粉,虽然底物较对照组丰富,但两者微生物多样性均有降低,推测可能是由于寺河矿区为无烟煤,富集时采用煤也为无烟煤,所以得到菌群对无烟煤有更好的适应性,而采用褐煤做底物或者是基本培养基培养时,微生物菌群适应性降低,所以菌群多样性下降。
表1 样品间Alpha多样性统计
Table 1 Alpha diversity of bacterial communities at different fermentation stages
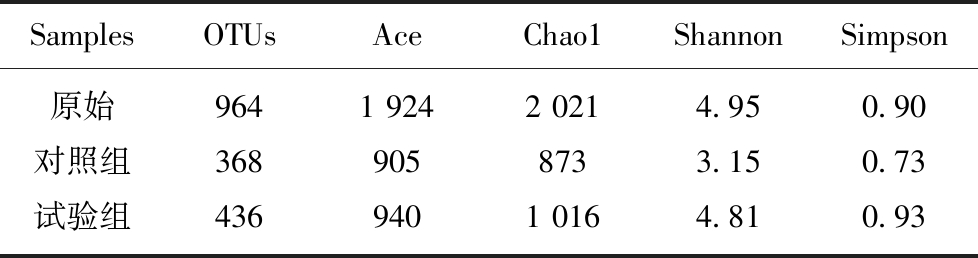
图2和3分别为原始和试验及对照组产气后菌群在门和属水平的群落组成分析。由图2可知褐煤生物产气所用原始微生物中主要优势菌门包括厚壁菌门(Firmicutes,27%)、WWE1(25%)、拟杆菌门(Bacteroidetes,21%)、互养菌门(Synergistetes,13%)以及丰度较低的变形菌门(Proteobacteria,5%)和绿弯菌门(Chloroflexi,1%)。经过一段时间的培养后微生物门类结构发生明显变化,其中试验组微生物中主要优势菌门主要为厚壁菌门(Firmicutes,40%)、变形菌门(Proteobacteria,19%)、WWE1(13%)、拟杆菌门(Bacteroidetes,8%)、互养菌门(Synergistetes,6%)和少量的脱铁杆菌门(Deferribacteres,2%);而对照组中微生物优势门类则减少为3类,依次为厚壁菌门(Firmicutes,73%)、变形菌门(Proteobacteria,22%)和拟杆菌门(Bacteroidetes,3%)。从属水平上群落丰度分析可以发现,在原始微生物中W22(38%)是该富集样本中丰度最高的微生物,其次为Proteiniclasticum(21%)和VadinCA02(18%)。经褐煤生物产气培养后的试验组中W22(22%)依然保持优势地位,脱硫弧菌属 Desulfovibrio(22%)也占有相同比重,其次为Tissierella_Soehngenia(11%)和VadinCA02(10%)(b)。可见,褐煤富集会对原始菌群的组成产生影响,原始菌群中的Proteiniclasticum相对丰度急剧减少,而脱硫弧菌属 Desulfovibrio 和Tissierella_Soehngenia在原始菌群中可能丰度较低均没有检测到,但是产气后其丰度明显上升。对照组中丰度最高的微生物则为厚壁菌门中的Tissierella_Soehngenia(58%),其次为Clostridium(16%)(c)。
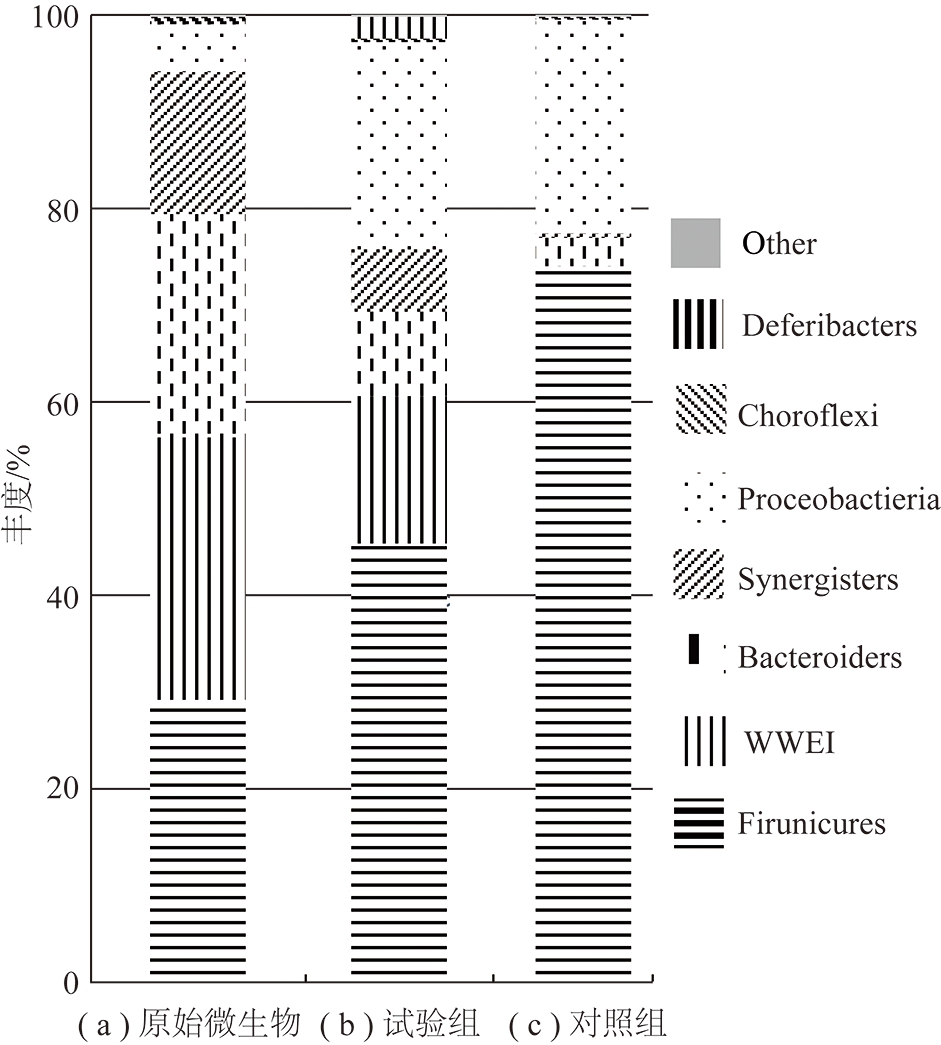
图2 原始微生物、试验组、对照组在门水平多样性分析
Fig.2 Biodiversity analysis of original microorganism, experimental group and control group at phyla level
厚壁菌门在3组样品中占比最大,其中的微生物主要参与一些混合酸、醇和中性物质的生成。其中的Proteiniclasticum和梭菌属(Clostridium)均属于梭菌科参与一些纤维质、几丁质等水解过程[17]。Tissierella菌属可以利用有机物代谢产生乙酸、丁酸、氨氮、CO2 等物质[18]。互养单胞菌(Syntrophomonas)、醋杆菌属(Anaerovorax)等在降解多糖、脂肪酸等有机物、产氢产酸等过程中扮演重要角色[19-21]。未培养WWE1菌门在产甲烷混合菌群中常被检测到,在降解烃类和纤维素等物质过程中发挥重要作用[22-23],可能具有将丙酸氧化为乙酸和 CO2 的能力[24]。WWE1菌门中的W22其具体功能还未见文献报道,有待进一步研究。拟杆菌门是一类化能自养型微生物,样品中属于该门的微生物主要有产电菌(Dysgonomonas)和产酸菌(Paludibacter),它们可能参与苯酚等有机物的降解产酸等过程,在微生物燃料电池等反应器中也有发现报道[25-26]。变形菌门物种丰富,具有极为广泛的代谢类型,其中脱硫弧菌属(Desulfovibrio)为硫酸盐还原菌,可将硫酸盐还原为H2S,为微生物提供能量,减轻对甲烷菌的竞争性抑制和毒害作用[27]。检测到的柠檬酸杆菌属(Citrobacter)具有降解长链烷烃、还原硫酸盐等功能[28-29],互营菌属(Syntrophus)常在降解石油烃等富集物中被检测到,具有降解烷烃、长链脂肪酸等功能[30-31]。地杆菌(Geobacter)是隶属变形杆菌δ亚纲的Fe(Ⅲ)还原细菌,广泛分布于厌氧沉积环境中,具有代谢乙酸盐的特征[32]。样品中属于互养菌门中的微生物分别有胺小杆菌属(Aminobacterium)、产氢产乙酸菌属(Sedimentibacter),它们是厌氧系统中重要的氨基酸降解和产氢产乙酸的功能细菌,该类微生物在连接水解发酵过程与产甲烷过程中起重要作用[33-34]。脱铁杆菌门是一类通过专性或兼性厌氧代谢获得能量的细菌,可利用多种电子受体,在试验组中检测到较低丰度的菌群[35]。VadinCA02和VadinHB04在原始和试验组样品中丰度较高,有研究表明在废水处理器中常被检测到,在有机物降解过程中发挥重要作用[36-37]。
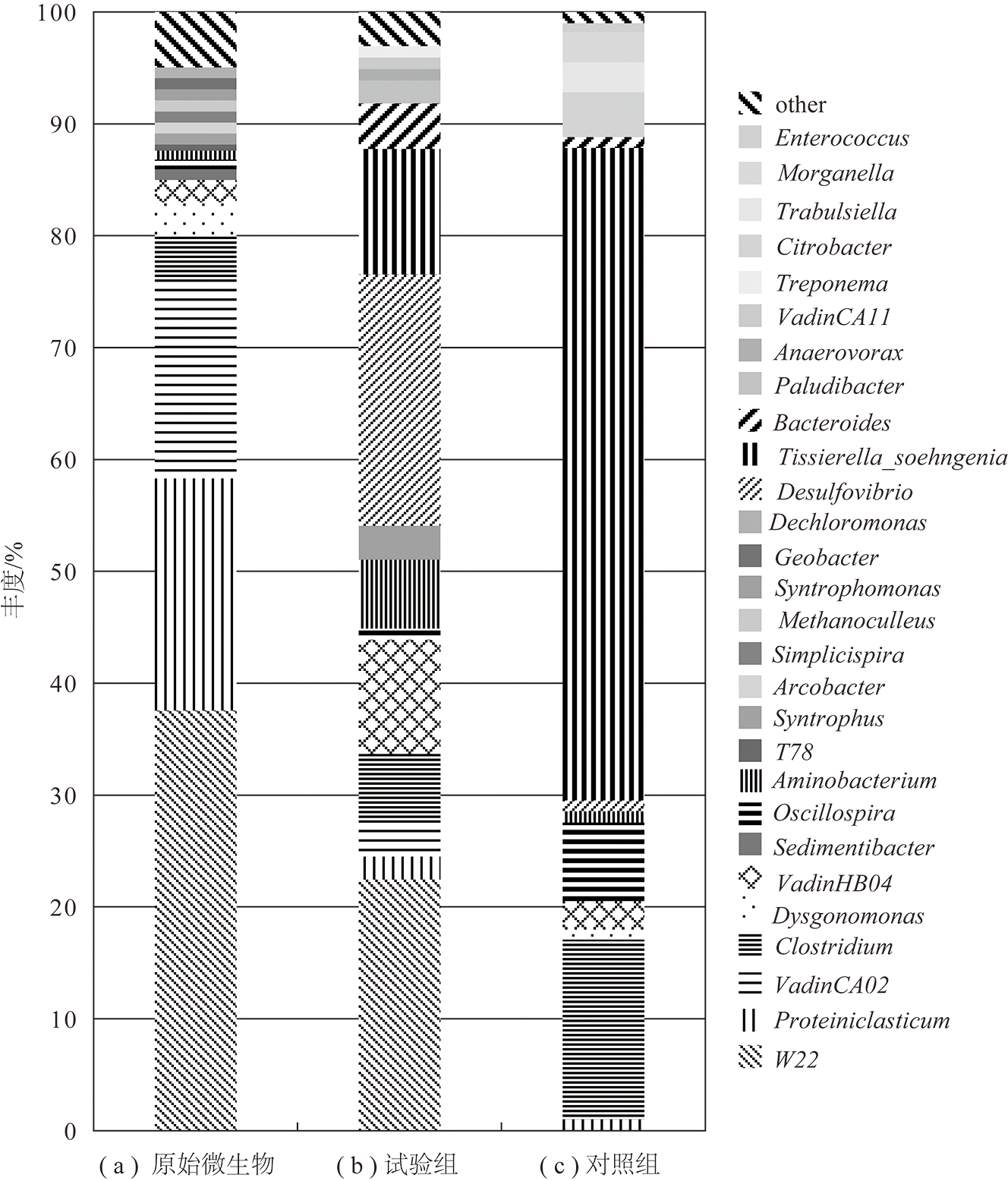
图3 原始微生物、试验组、对照组在属水平多样性分析
Fig.3 Biodiversity analysis of original microorganism, experimental group and control group at genus level
原始微生物中古菌主要为广古菌门(Euryarchaeota)中的甲烷囊菌属Methanoculleus(1%),而培养后的试验组中古菌主要为瘤胃古菌中的VadinCA11(1%)。Methanoculleus属于甲烷微菌球纲,该纲是目前产甲烷古菌研究报道最多的微生物,可以利用 H2/CO2、甲酸盐等小分子产生甲烷[38-39]。
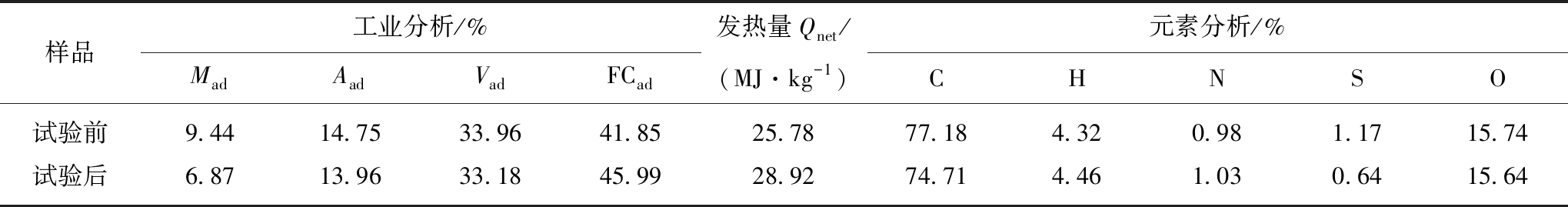
胜利褐煤基本理化性质分析见表2,煤样挥发分较高为33.96%,具有富氢、富氧等基本特征。对比试验前后煤样理化特性可知,经微生物降解作用后,水分、灰分挥发份均降低,固定碳百分比升高,在脱硫菌、产氢产乙酸菌等的作用下,煤样H/C升高,S,O元素比例下降,说明煤中易被微生物利用的短链和侧链被降解,剩下复杂的高分子结构难以降解。
表2 褐煤工业分析和元素分析结果
Table 2 Lignite industry analysis and elemental analysis results
原煤样品的扫描电镜结果(图4(a))表明煤表面结构松散,有明显的裂隙,有的裂隙大于10 μm有利于微生物附着降解,适宜进行生物产气模拟试验。生物产气试验后,观察煤样表面(图4(b))出现大量不同细胞形态的微生物结构,其中最丰富的是形态为1.0~1.5 μm短杆状和球状细胞,部分稍弯曲,主要位于煤表面较平整的区域。因厚壁菌可以产生芽孢,可以抵抗脱水和极端环境,结合测序结果分析可能是厚壁菌门的Tissierella_Soehngenia,Clostridium和Desulfovibrio等。同时还有一些形态较为特别的类型,比如直径约0.2 μm,长度为15 μm甚至更长更细的杆状结构,形态为3~5 μm的链球状结构等等,这些丝状结构的微生物有可能与微生物之间形成的纳米导线相关,其具体形成及其作用还有待进一步研究[40]。

图4 褐煤原煤样品和生物产气后样SEM照片
Fig.4 SEM images of lignite and after biogas production
(1)寺河矿区的煤层水富集微生物可以利用胜利褐煤进行产气,其产气周期为49 d,期间累计产甲烷量为83.1 mL,净产甲烷率为7.84 mL/g煤。
(2)测序结果表明,褐煤产气微生物门类主要集中在厚壁菌门、WWE1、互养菌门、变形菌门、拟杆菌门和广古菌门。原始微生物群落结构多样性较高,经褐煤和基本培养基产气后,群落多样性降低。其中W22,Proteiniclasticum,VadinCA02,Tissierella_Soehngenia,Clostridium,Desulfovibrio等菌属在褐煤产气过程中发挥重要功能。
(3)褐煤在生物产气作用后,水分、灰分、挥发分均降低,固定碳百分比升高,H/C升高,S元素比例下降,利用扫描电镜观察表面附着大量短杆状和球状细胞结构,并存在类似微生物纳米导线结构。
参考文献:
[1] 王爱宽.褐煤本源菌生气特征及其作用机理[D].徐州:中国矿业大学,2010.
WANG Aikuan.Liveness characteristics and mechanism of lignite indigenous bacteria[D].Xuzhou:China University of Mining and Technology,2010.
[2] STRAPOC D,MASTALERZ M,DAWSON K,et al.Biogeochemistry of microbial coal-bed methane[J].Annual Review of Earth and Planetary Sciences,2011,39:617-656.
[3] AHMED M,SMITH J W.Biogenic methane generation in the degradation of eastern Australian Permian coals[J].Organic Geochemistry,2001,32(6):809-816.
[4] ROBBINS S J,EVANS P N,ESTERLE J S,et al.The effect of coal rank on biogenic methane potential and microbial composition[J].International Journal of Coal Geology,2016,154-155:205-212.
[5] TANG Y Q,JI P,LAI G L,et al.Diverse microbial community from the coal beds of the Ordos Basin,China[J].International Journal of Coal Geology,2012,90-91:21-33.
[6] 王保玉,陈林勇,邰超,等.外源菌群煤生物气化初步研究:菌群结构,煤种及煤孔(裂)隙[J].煤炭学报,2014,39(9):1797-1801.
WANG Baoyu,CHEN Linyong,TAI Chao,et al.A preliminary study of biological coal gasification by exogenous bacteria:Microbiome composition,coal type,pore and seam fracture[ J].Journal of China Coal Society,2014,39(9):1797-1801.
[7] HE H,HAN Y X,JIN D C,et al.Microbial consortium in a non-production biogas coal mine of eastern China and its methane generation from lignite[J].Energy Sources,Part A:Recovery,Utilization,and Environmental Effects,2016,38(10):1377-1384.
[8] DARIUSZ S,FLYNN W P,COURTNEY T,et al.Methane-producing microbial community in a coal bed of the Illinois Basin[J].Applied and Environmental Microbiology,2008,74(8):2424-2432
[9] INAGAKI F,HINRICHS K U,KUBO Y,et al.Exploring deep microbial life in coal-bearing sediment down to~2.5 km below the ocean floor[J].Science,2015,349(6246):420-424.
[10] MAYUMI D,MOCHIMARU H,TAMAKI H,et al.Methane production from coal by a single methanogen[J].Science,2016,354(6309):222-225.
[11] DAWSON K S,STRAPOC D,HUIZINGA B,et al.Quantitative fluorescence in situ hybridization analysis of microbial consortia from a biogenic gas field in Alaska’s Cook inlet basin[J].Applied and Environmental Microbiology,2012,78(10):3599-3605.
[12] DONALD A K,ROMEO M F,CHRISTOPHE V.Molecular sequences derived from Paleocene Fort Union Formation coals vs.Associated produced waters:Implications for CBM regeneration[J].International Journal of Coal Geology,2008,76:3-13.
[13] GUO H G,YU Z S,ZHANG H X,et al.Pyrosequencing reveals the dominance of methylotrophic methanogenesis in a coal bed methane reservoir associated with Eastern Ordos Basin in China[J].International Journal of Coal Geology,2012,93:56-61.
[14] VICK S H W,TETU S G,SHERWOOD N,et al.Revealing colonisation and biofilm formation of an adherent coal seam associated microbial community on a coal surface[J].International Journal of Coal Geology,2016,160-161:42-50.
[15] 陈林勇,王保玉,邰超,等.无烟煤微生物成气中间代谢产物组成及其转化[J].煤炭学报,2016,41(9):2305-2311.
CHEN Linyong,WANG Baoyu,TAI Chao,et al.Composition and conversion of intermediate products in the process of anthracite gasification by microorganism[J].Journal of China Coal Society,2016,41(9):2305-2311.
[16] 谢家仪,董光军,刘振英.扫描电镜的微生物样品制备方法[J].电子显微学报,2005,24(4):440.
XIE Jiayi,DONG Guangjun,LIU Zhenying.Microbial sample preparation for SEM[J].Journal of Chinese Electron Microscopy Society,2005,24(4):440.
[17] COLOSIMO F,THOMAS R,LLOYD J R,et al.Biogenic methane in shale gas and coal bed methane:A review of current knowledge and gaps[J].International Journal of Coal Geology,2016,165:106-120.
[18] HARNS C,SCHLEICHER A,COLLINS M D,et al.Tissierella creatinophila sp.nov.,a gram-positive,anaerobic,non-spore-forming,creatinine-fermenting organism[J].International Journal of Systematic Bacteriology,1998,48:983-993.
[19] IMACHI H,SAKAI S,KUBOTA T,et al.Sedimentibacter acidaminivorans sp.nov.,an anaerobic,amino acids-utilizing bacterium isolated from marine subsurface sediment[J].International Journal of Systematic & Evolutionary Microbiology,2016,66(3):1293-1300.
[20] 韩睿,陈来生,李莉,等.PCR-DGGE研究青海农村户用沼气池微生物群落结构[J].中国环境科学,2015,35(6):1794-1804.
HAN Rui,CHEN Laisheng,LI Li,et al.PCR-DGGE study on microbial community structure of household biogas digesters in Qinghai rural areas[J].China Environmental Science,2015,35(6):1794-1804.
[21] ZHANG C,LIU X X.Syntrophomonas curvata sp.nov.an anaerobe that degrades fatty acids in co-culture with methanogens[J].International Journal of Systematic & Evolutionary Microbiology,2004,54(3):969-973.
[22] LIMAM R D,CHOUARI R,MAZEAS L,et al.Members of the uncultured bacterial candidate division WWE1 are implicated in anaerobic digestion of cellulose[J].Microbiologyopen,2014,3(2):157-167.
[23] 麻婷婷,承磊,郑珍珍,等.不同pH缓冲液对由乙酸产甲烷菌群结构的影响[J].微生物学报,2014,54(12):1453-1461.
MA Tingting,CHENG Lei,ZHENG Zhenzhen,et al.Effect of different pH buffers on the structure of methanogenic methanotrophs in acetic acid[J].Chinese Journal of Microbiology,2014,54(12):1453-1461.
[24] BERLENDIS S,LASCOURREGES J F,SCHRAAUWERS B,et al.Anaerobic biodegradation of BTEX by original bacterial communities from an underground gas storage aquifer[J].Environmental Science & Technology,2010,44(9):3621-3628.
[25] 王丽丽.可降解苯酚的高效产电菌株的分离筛选及生物学特性研究[D].哈尔滨:哈尔滨理工大学,2017.
WANG Lili.Isolation,screening and biological characteristics of highly producing strain of degradable phenol[D].Harbin:Harbin University of Science and Technology,2017.
[26] 尹亚琳,高崇洋,赵阳国,等.好氧-厌氧混合污泥启动微生物燃料电池产电性能及微生物群落动态特征[J].微生物学报,2014,54(12):1471-1480.
YIN Yalin,GAO Chongyang,ZHAO Yangguo,et al.Electricity generation and dynamics characteristics of microbial community of microbial fuel cells started up with mixture of aerobic/anaerobic sludge[J].Acta Microbiologica Sinica,2014,54(12):1471-1480.
[27] 宋燕莉.赵庄矿区煤层水产气菌群计数和物种分类分析[J].能源与节能,2016(11):51-53.
SONG Yanli.Counting and species classification analysis of aquatic gas-producing bacteria in coal seam of Zhaozhuang Mining Area[J].Energy and Energy Conservation,2016(11):51-53.
[28] 杨丽平,郑小红,曾国驱,等.1株具硫酸盐还原功能的柠檬酸杆菌的分离及其生理特性研究[J].环境科学,2010,31(3):815-820.
YANG Liping,ZHENG Xiaohong,ZENG Guoqu,et al.Isolation and characterization of a sulfate reducing Citrobacter sp.strain SR3.[J].Environmental Science,2010,31(3):815-820.
[29] 孙晶,宋东辉,刘凤路,等.一株烷烃降解细菌的分离鉴定及其降解特性[J].天津科技大学学报,2016,31(5):19-24.
SUN Jing,SONG Donghui,LIU Fenglu,et al.Isolation,identification and degradation characteristics of an alkane degradation bacterium[J].Journal of Tianjin University of Science & Technology,2016,31(5):19-24.
[30] 丁晨,承磊,何乔,等.互营烃降解菌系M82的脂肪酸降解特性[J].微生物学报,2014,54(11):1369-1377.
DING Chen,CHENG Lei,HE Qiao,et al.Fatty acid degradation characteristics of M82,an inter-hydrocarbon degradation strain[J].Chinese Journal of Microbiology,2014,54(11):1369-1377.
[31] TISCHER K,KLEINSTEUBER S,SCHLEINITZ K M,et al.Microbial communities along biogeochemical gradients in a hydrocarbon-contaminated aquifer[J].Environmental Microbiology,2013,15(9):2603-2615.
[32] 朱超,STEFAN Ratering,曲东,等.短期淹水培养对水稻土中地杆菌和厌氧粘细菌丰度的影响[J].生态学报,2011,31(15):4251-4260.
ZHU Chao,STEFAN Ratering,QU Dong,et al.Effect of short-term flooding culture on the abundance of Geobacillus and anaerobic viscous bacteria in paddy soil[J].Chinese Journal of Ecology,2011,31(15):4251-4260.
[33] HAMDI O,BEN H W,POSTEC A,et al.Aminobacterium thunnarium sp.nov.,a mesophilic,amino acid-degrading bacterium isolated from an anaerobic sludge digester,pertaining to the phylum Synergistetes.[J].International Journal of Systematic & Evolutionary Microbiology,2015,65(Pt 2):609-14.
[34] 占迪,何环,廖远松,等.褐煤强化产甲烷菌群的群落分析及条件优化[J].微生物学报,2018,58(4):684-698.
ZHAN Di,HE Huan,LIAO Yuansong,et al.Community analysis and optimization of conditions for intensified methanogens in lignite[J].Acta Microbiologica Sinica,2018,58(4):684-698.
[35] BUDWILL K,KOZIEL S,VIDMAR J.Advancements in underst-anding and enhancing biogenic methane production from coals[A].Canadian Unconventional Resources Conference[C].Canada:Society of Petroleum Engineers,2011:1-9.
[36] MOTTERAN F,BRAGA J K,SILVA E L,et al.Kinetics of methane production and biodegradation of linear alkylbenzene sulfonate from laundry wastewater[J].Environmental Letters,2016,51(14):1288-1302.
[37] 焦雅楠.印染废水与生活污水中抗生素抗性基因分布差异及机理研究[D].杭州:浙江大学,2017.
JIAO Yanan.Differences in the distribution of antibiotic resistance genes in printing and dyeing wastewater and domestic wastewater and their mechanisms[D].Hangzhou:Zhejiang University,2017.
[38] 王保玉,刘建民,韩作颖,等.产甲烷菌的分类及研究进展[J].基因组学与应用生物学,2014,33(2):418-425.
WANG Baoyu,LIU Jianmin,HAN Zuoying,et al.Recent progress and classification of methanogens[J].Genomics and Applied Biology,2014,33(2):418-425.
[39] 承磊,郑珍珍,王聪,等.产甲烷古菌研究进展[J].微生物学通报,2016,43(5):1143-1164.
CHENG Lei,ZHENG Zhenzhen,WANG Cong,et al.Recent advances in methanogens[J].Microbiology China,2016,43(5):1143-1164.
[40] 刘茜,马旅雁.微生物纳米导线,纳米管与胞外电子传递及胞间物质交换[J].矿物岩石地球化学通报,2018,37(1):48-54.
LIU Xi,MA Lüyan.Microbial nanowire and nonotube and their improvements in extracellular electron transfer and exchange of intracellular molecular,Bulletin of mineralogy,petrology and Geochemistry,2018,37(1):48-54.