自20世纪30年代始,微纳尺度下的颗粒气泡黏附就引起了学者的广泛关注并逐渐涌现出一系列试验技术用于研究颗粒气泡间微纳作用力及液膜薄化破裂动力学过程,其总体上可以分为两类:力测量法及排液法。力测量法是借助原子力显微镜(AFM)等技术手段测试颗粒气泡间相互作用力,通过流体力学排液模型计算液膜薄化动力学行为;排液法则是通过光学显微干涉技术直接获得气液界面变形及排液动力学数据,通过耦合扩展DLVO理论及排液方程求解相互作用力信息,如单气泡撞板显微干涉技术、薄膜压力平衡技术及表面力分析仪(SFA)等。2015年,SHI等[1]通过AFM与反射干涉对比显微镜(RICM)联用首次实现了力、气泡变形及液膜云图的同步测试,直接证明了力测试与排液法二者之间的理论统一。在宏观尺度下颗粒气泡黏附研究进展的基础上,系统的对微纳尺度下颗粒气泡间相互作用力及液膜薄化破裂动力学试验技术研究进展进行综述。
1 排液法
1.1 单气泡撞板显微干涉技术
在气泡接近固体平坦基板的过程中,中间液膜的厚度可以通过薄膜干涉技术获得。在膜厚度数据的基础上可计算颗粒气泡间分离压力。需要特别指出的是,当基板-气泡间相互作用力为排斥力(即排斥性分离压力)时,液膜最终会到达热力学平衡状态,此时液膜分离压力等于气泡内部拉普拉斯压力。因此,可以通过改变气泡的半径来获得不同膜厚度下的分离压力。然而这种方法仅对排斥性分离压力有效,当颗粒气泡间作用力表现为引力时,液膜是不稳定的,表面力只能通过动态排液法来确定。使用高速动态摄像机记录膜薄化干涉条纹序列,在总曲率压力中扣除流体压力项获得分离压力信息。
单气泡撞板的一种情形是通过在毛细管末端生成一固定尺寸气泡并驱动气泡逼近矿物基板。DERJAGUIN 和 KUSAKOV[2]使用单色光显微干涉技术研究了气泡-亲水玻璃基板间的相互作用力,通过改变气泡直径绘制亲水玻璃基板与气泡间的分离压力等温线,即DLVO理论的雏形。其中,膜厚度h计算公式为
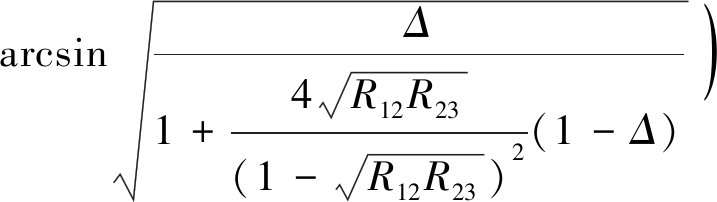
(1)
式中,![]() 为空气,水及固体基板的折射率;I,Imax,Imin分别为瞬时光强,最大反射光强,最小反射光强,cd;λ为波长,m;m为干涉条纹级数,取整数。
为空气,水及固体基板的折射率;I,Imax,Imin分别为瞬时光强,最大反射光强,最小反射光强,cd;λ为波长,m;m为干涉条纹级数,取整数。
单色光显微干涉的局限在于颗粒气泡间绝对分离距离的确定,具体表现在牛顿环求解时如何准确确定干涉条纹的色序。YOON和YORDAN[3]引入了双波长干涉技术测试了甲基化石英表面润湿膜的临界破裂厚度,发现临界膜破裂厚度随甲基化程度的增加而增加。与此同时,亲水石英表面的润湿膜随十二胺盐酸盐DAH的加入同样变得不稳定,当DAH浓度超过其临界胶束浓度时,不稳定膜再次向稳定液膜转变,与诱导时间测试结果保持一致[4]。GAO和PAN[5]进一步使用三波长反射干涉显微镜系统研究了气泡尺寸对颗粒气泡间润湿膜排液的影响,三波长反射干涉显微镜系统如图1所示。结果发现三波长干涉技术在确定液膜厚度时无需额外的物理假设,在200 nm液膜厚度范围以内的测试误差小于1 nm,试验发现液膜临界破裂厚度与气泡尺寸呈正相关关系,气泡尺寸越小临界破裂厚度越小。

图1 三波长反射干涉显微镜系统[5]
Fig.1 Photo and schematic representation of the triwavelength reflection interferometry microscope[5]
KARAKASHEV等[6]借助Image J软件实现了亲水性玻璃与气泡间润湿膜的三维空间重构,探索了玻璃基板切向移动速度对润湿膜时空云图分布的影响,试验系统如图2所示。基板的切向运动会引入额外的升浮压力使得气液界面进一步远离玻璃表面,但在上述复杂条件下,气泡表面轮廓会呈现非轴对称分布,这无疑会增加单色光干涉确定颗粒气泡间液膜厚度的难度,表面力信息的提取同样会变得更加复杂。

图2 润湿膜三维呈像系统[6]
Fig.2 Experimental setup for 3D imaging of wetting film[6]
另外一种情形则主要是针对自由气泡上浮撞板系统。PARKINSON和RALSTON[7]研究了上浮气泡与亲水性二氧化钛之间的液膜薄化动力学及分离压力。气泡在上浮过程中因流体阻力会产生弹性压扁甚至是涟漪变形。因此,在PARKINSON的试验中,使用了微米级气泡(直径小于100 μm)来避免流体弹性动力压扁效应,同时微泡小的终端升浮末速可进一步减小流体阻力。无边界滑移条件下的Taylor模型可准确描述液膜薄化动力学。HENDRIX等[8]进一步使用常规毫米级气泡替换微米气泡,Stokes-Reynolds-Young-Laplace(SRYL)模型可以很好的预测气泡表面“酒窝”的形成和后续的排液动力学。MANICA等[9-10]在原有理论基础上重新构建了用于描述毫米气泡“撞板-回弹”过程中界面变形及液膜薄化的理论框架,将浮力、流体阻力及附加质量力加入到力平衡方程中。然而如此宽尺度雷诺数条件下的界面边界条件的确定仍需进一步研究。自由气泡上浮技术的主要缺点是试验过程中难以实现气泡运动轨迹的精确控制,一方面要求颗粒气泡碰撞点发生在相机视场范围内;另一方面,上置高速相机精准调焦也存在着较大技术困难。
1.2 薄膜压力平衡技术
薄膜压力平衡技术最早用于绘制泡沫膜分离压力等温线[11-14]。其核心部件为Scheludko槽,膜厚度由光学干涉法确定。美国弗吉尼亚理工大学PAN和YOON[15]对传统Scheludko槽的中心排液模式进行了改进并将固体基板直接置于改进后的Scheludko槽顶部使其适用于润湿膜排液研究,其中的一大优点是可以实现非光学透明固体表面润湿膜稳定性研究,改进后的薄膜压力平衡系统如图3所示。同样的,对于排斥性膜分离压力等温线可以直接通过改变毛细压力获得(外界气氛控制或变化Scheludko槽直径);而对于吸引性的分离压力,必须通过记录液膜薄化破裂动力学过程来确定。PAN和YOON[14]记录了气泡与不同接触角金板表面的润湿膜排液行为。试验中起始膜厚度固定在350 nm,液膜半径固定在70 μm。当金板的后退接触角为17°时,20 s后液膜最终达到80 nm厚的平衡状态;而当接触角增加至79°时,液膜在1.26 s时间内在80 nm临界厚度处破裂,疏水引力是造成液膜快速薄化并破裂的根本原因。使用Stefan-Reynolds平坦膜排液方程对液膜障碍环处的薄化数据进行拟合得到疏水金板与气泡间疏水力常数为2×10-17 J,大于体系Hamaker常数3个数量级。特别指出的是该试验首次实现了颗粒气泡体系疏水力的定量表征,但改进的Scheludko槽中液膜内流场可能呈非对称分布,影响测试结果准确性。

图 3 改进后的薄膜压力平衡系统[15]
Fig.3 Schematic of the modified TFB apparatus[15]
PAN等[16]进一步在膜排液方程中加入了气液界面曲率压力项,同时考察了疏水界面的边界滑移效应对膜薄化速率的影响,发现疏水界面边界滑移难以诱发如此快的排液速率。使用单指数衰减疏水力模型对试验数据进行拟合发现疏水金板与气泡间的疏水力是一种长程作用力,其衰减长度高达36 nm。虽然目前关于疏水作用力的起源还没有达成共识,此长程作用力可能是由于固液界面纳米气泡成核或表面杂质污染引起。
1.3 表面力分析仪
表面力分析仪(SFA)最早用于测试气相或液相下光滑云母间的相互作用力,通过白光干涉获得等色序干涉条纹获得表面分离距离,其中下端样品与力弹簧片相连测试作用力[17-19]。与传统单色光干涉相比,白光干涉在没有已知参考距离的情况下可获得绝对分离距离,纵向分离距离分辨率可达0.1 nm。然而一般只有光学透明的光滑材料才能用于SFA测试。HORN 等[20-24]对传统SFA进行了改进使其适用于颗粒气泡间相互作用研究,改进后的SFA系统如图4所示。使用尾端封闭的毛细管代替下端云母片,白光干涉系统监视液膜薄化云图。然而改进后的SFA不再具备力测试功能,只能通过求解膜排液动力方程才可获得表面力及流体阻力。因此,从这个角度上讲,SFA仍属于排液法范畴。PUSHKAROVA和 HORN[22]发现气泡在靠近云母表面过程中,为了维持恒定的表面电势气液界面存在电荷调制现象。在短距离范围内,这种调制甚至会产生轻微的电荷反转使得排斥性静电力变为吸引力,范德华力是最终稳定性液膜中的唯一排斥力。CASTILLO等[25]使用SFA进一步研究了云母表面润湿膜中表面力与流体阻力的耦合作用,在去离子水中,经典DLVO理论可以很好的预测准静态接近过程中的长程排斥力,但液膜并没有在此斥力作用下到达平衡状态,相反观测到了一个轻微的跳入现象,中心液膜进一步靠近而外围则形成两个“肩膀”。在1 mmol/L KCl溶液中同样观察到了类似的液膜薄化行为。SRYL模型难以预测最终膜云图中两个“肩膀”的形成。气液界面电荷移动可以很好的解释这种现象,在膜排液过程中,存在着一个由无滑移向滑移界面转变的过渡点。正如前文所述,改进后的SFA仍然无法实现非透明矿物与气泡间的液膜薄化动力学测试。

图4 改进后SFA系统[26]
Fig.4 Aschematic of the modified SFA[26]
2 力测量法
AFM是测试颗粒气泡间相互作用力的最常用手段。历史上,苏黎世联邦实验室BINNIG等[27]发明了第1台AFM用于纳米尺度下非导电样品的表面形貌成像。与传统扫描隧道显微镜相比,AFM对样品材料及成像环境的要求相对宽松,应用也因此更加广泛。AFM利用微悬臂针尖感受并放大与样品间的微弱作用力,通过反馈回路系统保持探针以“恒力”或“恒高”模式进行光栅扫描从而获得样品表面的三维形貌图,如图5所示。除了最原始的成像功能,AFM也越来越多的被用于测试表面力、摩擦力、黏附力及流体力等。借助光学杠杆系统将力引起的微悬臂形变直接转化为四象限光强检测器的电压信号,根据胡克定律获得作用力信息。目前,大部分商业化AFM均具有力谱(力曲线)测试功能,但针尖的不规则形状无疑增加了作用力定量表征的难度。1991年,DUCKER等[28-29]和BUTT[30]将微米级玻璃/石英小球黏附于微悬臂末端制备了“胶体探针”,胶体探针AFM也逐渐成为表界面领域内相互作用力测试的强有力工具。与SFA相比,AFM对样品配置更加灵活,同时小的相互作用面积使得样品污染问题得到了有效的解决[31]。

图5 AFM工作示意[53]
Fig.5 Schematic of AFM[53]
在浮选领域,AFM同样可广泛应用于研究矿物表界面特征[32]、药剂吸附[33-38]、颗粒间相互作用力[39-45]及颗粒气泡间相互作用机制[46-51]。例如,XING等[52]借助胶体探针AFM研究了不同类型黏土矿物(高岭石与蒙脱石)与煤基板间的相互作用力,发现在去离子水条件下煤与高岭石间作用力为斥力,而蒙脱石与煤间则体现为引力。随着钙离子的加入,吸引力增加,浮选过程中细泥罩盖现象加剧。XING等[38]同样测试了氧化煤颗粒与石蜡和硬脂酸基板间的相互作用力,结果表明:氧化煤与硬脂酸之间的引力作用是脂肪酸类药剂强化氧化煤浮选的根本原因,极性脂肪酸可以在氧化煤表面亲水位点完成吸附进而提高氧化煤可浮性。XING等[53]曾对AFM在矿物浮选领域的应用进行了系统的综述。
与排液法相比,AFM可以直接获得颗粒气泡间相互作用力。1994年,DUCKER等[54]和BUTT[55]首次使用AFM测试了颗粒气泡间相互作用力。DUCKER等[54]发现到亲水性颗粒与气泡间存在着吸引力,这可能是由于样品准备过程中的污染问题;BUTT[55]则发现只有在疏水性颗粒与气泡间才存在引力,当引力梯度超过微悬臂弹性系数时发生跳入黏附现象。
AFM所获得的原始数据是力-位移(扫描管Z轴伸缩量)曲线而非力-分离距离曲线。分析AFM力曲线最重要的步骤是如何准确确定零距离以获得力-距离曲线,零距离定义为力-位移曲线中探针与样品表面的绝对分离距离为零的点。在硬接触系统中,零距离可以通过原始力-位移曲线中的线性接触区域来确定。对于颗粒气泡相互作用系统,由于气泡表面的变形效应,很难找到硬接触点。近年来学者们就如何确定颗粒气泡间分离距离做了广泛的探索。DUCKER等[54]认为在外部压力下气泡可以近似看作为一根线性弹簧,胶体探针-气泡系统可以看作是两根胡克弹簧的串联组合,力曲线线性接触区斜率的倒数等于气泡弹性系数与微悬臂本身弹性系数的倒数之和。基于这种假设,PREUSS和BUTT[50,56]系统研究了十二烷基硫酸钠(SDS)和十二烷基三甲基溴化铵(DTAB)对亲水玻璃微球气泡间相互作用力的影响,发现阴离子型SDS的加入会进一步增加斥力作用,而随着阳离子型DTAB的加入会诱发黏附,DTAB极性头基与玻璃表面发生键合使得其变得疏水产生疏水力。当DTAB浓度超过其临界胶束浓度时会形成双层吸附,排斥力再次主导颗粒气泡相互作用。
NGUYEN等[57-58]研究了接近速度对颗粒气泡相互作用的影响,亲水性玻璃小球与气泡间的排斥作用力随着碰撞速度的增加而增加,当速度小于0.6 μm/s时,流体阻力的贡献可以忽略而表面力则起主导作用;当速度进一步增加时,斯托克斯方程可以很好的预测长程范围内的斥力行为。尽管如此,气泡在外部压力的作用下并非一直遵守线性响应,气泡弹簧假设不能获得整个作用区域内的界面轮廓和液膜排液动力学。另一方面使用胶体探针AFM测试颗粒气泡间相互作用需要试验人员具备精致的试验技巧,通过步进电机和Z轴扫描管的组合获得完整的力曲线。
GILLIES等[59-60]指出软物质在足够小的作用力下可以表现出与坚硬表面类似的力学响应,长程范围内的弱静电力不足以使得气泡表面发生明显变形。基于此,通过拟合长程范围内的静电斥力获得了分离距离信息。TARAN等[61]借助此方法研究了气泡静置时间对亲水性石英小球与气泡相互作用的影响。但此方法的局限性在于需要单独测试颗粒气泡的表面电位,尤其是如何获得气泡表面电位仍存在一定挑战。
CHAN等[62-64]提出了一种基于力平衡的分析模型(CHAN-DAGASTINE-WHITE模型)用于预测胶体探针和变形界面相互作用。ENGLERT等[65]使用此模型研究了疏水性及表面电性对沥青颗粒和气泡间相互作用的影响。CHAN[66]进一步推导了增益型SRYL模型用于预测动态AFM试验中胶体探针与变形界面间相互作用力及液膜时空演化规律。通过SRYL模型拟合力曲线数据获悉作用过程中必要的物理信息。MANOR 等[67-68]使用AFM直接测试了气泡与固体基板间的相互作用力,非边界滑移条件下的SRYL模型很好的预测了液膜分离压力及液膜云图演化。与传统胶体探针技术不同的是,其将微米级气泡黏附与微悬臂末端(气泡探针)[69-72]。使用气泡探针的优点是气液界面属于绝对光滑表面,放宽了对固体材料的限制。传统胶体探针技术中,一般只有表面光滑的石英及玻璃小球等才能用于AFM试验。SHI等[73]则同样使用气泡探针技术测试了气泡与不同疏水性的OTS覆盖的云母间相互作用力,AFM下针速度恒定为0.1 μm/s。结果发现:当云母表面接触角为45°时,气泡在7.5 nm处发生跳入黏附。当额外考虑0.8 nm的疏水力衰减长度时,SRYL模型能够很好的拟合力曲线,如图6所示;当接触角增加至85°时,跳入黏附距离和疏水力衰减长度均呈现增加趋势,说明疏水力随着接触角的增加而增加。疏水分离压力是液膜破裂的主要原因。

图6 0.5 mol/L NaNO3溶液中80 μm气泡探针与45°接触角云母间相互作用[73]
Fig.6 Interaction between a bubble probe with radius of 80 μm approaching hydrophobized mica in 0.5 mol/L NaNO3 aqueous solution[73]
h为液漠厚度;r为液膜径向坐标
CUI等[74]进一步研究了溶液pH和盐离子浓度对疏水云母-气泡间疏水力的影响,疏水力衰变长度始终维持在1.0 nm左右,其大小与pH和盐离子浓度无关。XIE等[75-76]则测试了闪锌矿和辉钼矿与气泡间的黏附机理,在上述体系中同样发现了短程疏水力作用。需要指出的是在上述气泡探针AFM试验中所测到的疏水力作用程远远小于PAN等[15]使用薄膜压力平衡技术所获得的力作用程(36 nm衰减长度),造成这种差异的原因至今仍不明确。
3 力与液膜排液同步测试
3.1 AFM-RICM联用
随着光谱技术的发展,涌现出了众多新颖的AFM基光谱联用技术用于测试分离距离。TABOR等[77]搭建了激光共聚焦荧光-AFM联用系统,借助共聚焦的原位三维重构实现了微米油滴间的分离距离测量,试验测试值与理论计算值有着较高的吻合度,但气液界面的透镜效应限制了激光共聚焦在颗粒气泡系统中的进一步应用。美国卡耐基梅隆大学PRIEVE教授发明了全内反射荧光显微镜用于测量胶体离子和表面之间微弱相互作用[78],通过衰逝波散射光强记录布朗运动状态下胶体离子在固体平板上方的平均间距。CLARK等[79]利用全内反射技术搭建了衰逝波-AFM系统,借助衰逝波在固液界面垂直方向的衰减特性获得了胶体探针颗粒与基板间的分离距离,然而这种技术仅在200 nm分离距离内有效。同时气泡界面变形进一步增加了散射光强求解的难度。
SHI等[1]创新性地将RICM与AFM结合对气泡和云母基板间的相互作用力和液膜云图进行了同步测试。固定于微悬臂末端的微米级气泡在AFM步进电机和扫描管的协同配合下慢慢接近云母表面,AFM获得纳牛级分辨率的作用力,RICM则获得纳米级膜厚度。与传统显微干涉镜的不同在于RICM将界面本身的曲率耦合入光强计算公式中,尤其适用于微米级弯曲界面形状重构。最近,CONTRERAS-NARANJO和UGAZ[80]对复杂的RICM算法进行了改进使其能够适用于任意凸起物体的轮廓成像。
研究发现:在500 mmol/L氯化钠溶液下,气泡与亲水性云母间形成稳定性液膜,非边界滑移条件下的SRYL模型很好的预测了作用力与液膜的动态演化规律。当云母表面接触角增加至45°或90°,力曲线观测到了明显跳入黏附现象,同时,跳入黏附距离(临界液膜破裂厚度)随接触角的增加而增加。当在SRYL模型中考虑疏水力作用时,模型可以对膜排液动力学及临界膜破裂厚度做出很好的预测。试验发现疏水力衰变长度在1 nm左右范围,说明颗粒气泡间疏水力仅为一种短程作用力。
3.2 变形体系力分析仪
PAN和YOON[81]开发了一种适用于同步测试气泡-固体基板间相互作用力及液膜薄化的变形体系力分析仪(FADS),如图7所示。在FADS中,目的矿物可以直接溅射或黏附在自制的毫米宽微悬臂上,气泡则固定在疏水石英玻璃上。在悬臂接近气泡表面的过程中,其纤维激光干涉单元可直接测量悬臂的形变获得相互作用力,在下部配置的光学干涉单元测试动态液膜云图。与此同时,FADS配置了侧视相机记录悬臂与气泡作用过程中的动态接触角。与AFM-RICM不同的是,FADS对固体基板的材料限制更少,对于光学不透明的硅、金、银等矿物同样适用。另一方面,试验过程中同样可以更灵活的改变气泡尺寸,AFM-RICM体系下的气泡尺寸因受微悬臂本身的直径限制通常仅在100 μm范围左右。PAN和YOON[81]使用FADS研究了金板表面接触角对其表面润湿膜排液行为的影响,观察到了与薄膜压力平衡技术相一致的实验现象。亲水性金板表面润湿膜在排斥性膜分离压力下最终到达热力学平衡态;疏水性金板表面在长程疏水力的作用下在快速薄化并发生破裂。需要指出的是,Frumkin-Derjaguin等温线表明长程疏水力加速排液动力学而液膜的破裂是短程疏水力导致的。

图7 FADS工作示意[81]
Fig.7 Schematic of the FADS[81]
3.3 薄液膜力分析仪
对于AFM-RICM及FADS系统,其共同的缺点是仅仅能在低气泡雷诺数条件下(<10-2)进行试验。例如,AFM下针速率仅在0~50 μm/s变化。在实际浮选中,颗粒气泡碰撞接近速度远大于此。加拿大阿尔伯塔大学XU等开发了一种可以在中等气泡雷诺数条件下(10-2<Re<102)完成颗粒气泡间相互作用力测试的薄液膜力分析仪[82-86]。从结构组成看,薄液膜力分析仪(ITLFFA)是诱导时间测量仪和力测试仪的结合版。诱导时间测量仪的高频扬声器驱动气泡快速接近下方的石英基板,基板则固定于双晶电压片力学传感器上,整个液体池放置于倒置光学显微镜上借助双波长干涉模块获得液膜排液信息。ZHANG等[86]使用薄液膜力分析仪研究了1 mm/s接近速度下毫米级气泡与不同疏水性石英基板间的相互作用,接近过程中因为强的流体阻力气液界面形成了明显的凹陷。对于亲水性石英,凹陷膜最终到达平坦的平衡膜;对于疏水性石英,液膜在液膜障碍环处破裂,但并未实现对疏水力的定量求解。ZHANG等[87]进一步探索了逼近速度对亲水性石英表面润湿膜时空演化的影响,当逼近速度小于0.01 mm/s时,流体阻力不足以克服气泡内部拉普拉斯压力。
ZHANG等[88]进一步利用薄液膜力分析仪对不同疏水性固体表面的边界滑移状态进行了研究。不同疏水性石英基板与一毫米级气泡间的液膜演化云图如图8所示。结果发现固液界面边界滑移长度随着接触角的增加而增加,这种滑移现象产生的原因是由于固体表面的纳米粗糙度。同时对于特定的疏水性石英基板,存在着临界的接近速度诱发边界滑移。

图8 不同疏水性石英基板与一毫米级气泡间的液膜演化云图[88]
Fig.8 Evolution of spatiotemporal film thickness between a millimeter bubble and silica surface with different surface hydrophobicity[88]
3.4 浮选黏脱附微力测试仪
上述力与排液同步测试技术均属国外学者开发,国内学者因试验设备和技术的限制在颗粒气泡间相互作用力及液膜排液方面的研究鲜见报道。国家煤加工与洁净化工程技术研究中心一直致力于浮选黏脱附微力测试仪(FADFA)的研制开发。在现有浮选液膜分析仪的基础上,通过额外增加微力测试模块实现了颗粒气泡相互作用力和液膜薄化动力学的同步测试。与现有技术相比,浮选黏脱附微力测试仪在力测试性能方面显示出了更大的技术优势,在保证力学分辨率的前提下最大可能的扩大了力学量程。其中设备力学分辨率达0.5 nN,力学量程±200 mN。在实现黏附过程研究的同时,同样可实现后续的脱附单元研究,相关试验研究成果将在后续文章进行报道。
4 结 语
对微纳尺度下颗粒气泡间相互作用力及液膜薄化破裂动力学试验技术研究进展进行了综述。技术总体上可以分为两类:力测量法及排液法。排液法则是通过光学显微干涉技术直接获得气液界面变形及排液动力学数据,通过耦合扩展DLVO理论及排液方程求解相互作用力信息,如单气泡撞板显微干涉技术、薄膜压力平衡技术及SFA等。力测量法是借助AFM等技术手段测试颗粒气泡间相互作用力,通过流体力学排液模型计算液膜薄化动力学行为。随着AFM-RICM、FADS和ITLFFA等技术的问世,作用力和液膜排液的同步测试已经成为一种技术趋势,充分助力了浮选颗粒气泡黏附基础研究。但目前颗粒气泡体系疏水力的长程及短程来源机制仍不明确,基于现有研究进展,应进一步开展颗粒气泡间疏水力的系统研究,通过借助不同检测技术的优势互补及分子动力学模拟等手段,有望从根本上阐明这一科学问题。
[1] SHI C,CUI X,XIE L,et al.Measuring forces and spatiotemporal evolution of thin water films between an air bubble and solid surfaces of different hydrophobicity[J].ACS Nano,2015,9(1):95-104.
[2] DERJAGUIN B V,KUSAKOV M M.Acta Physicochim[J].URSS,1939,10(2):153-174.
[3] YOON R H,YORDAN J L.The critical rupture thickness of thin water films on hydrophobic surfaces[J].Journal of Colloid and Interface Science,1991,146(2):565-572.
[4] GU G X,XU Z X,NANDAKUMAR K,et al.Effects of physical environment on induction time of air-bitumen attachment[J].International Journal of Mineral Processing,2003,69:235-250.
[5] GAO Y,PAN L.Measurement of instability of thin liquid films by synchronized tri-wavelength reflection interferometry microscope[J].Langmuir,2018,34:14215-14225.
[6] KARAKASHEV S I,STOCKELHUBER K W,TSEKOV R,et al.Bubble rubbing on solid surface:Experimental study[J].Journal of Colloid and Interface Science,2013,412:89-94.
[7] PARKINSON L,RALSTON J.The interaction between a very small rising bubble and a hydrophilic titania surface[J].Journal of Physical Chemistry C,2010,114(5):2273-2281.
[8] HENDRIX M H W,MANICA R,KLASEBOER E,et al.Spatiotemporal evolution of thin liquid films during impact of water bubbles on glass on a micrometer to nanometer scale[J].Physical Review Letters,2012,108(24):247803.
[9] MANICA R,KLASEBOER E,CHAN D Y C.The impact and bounce of air bubbles at a flat fluid interface[J].Soft Matter,2016,12(13):3271-3282.
[10] MANICA R,KLASEBOER E,CHAN D Y C.Force balance model for bubble rise,impact,and bounce from solid surfaces[J].Langmuir,2015,31(24):6763-6772.
[11] KARAKASHEV S I,MANEV E D.Hydrodynamics of thin liquid films:Retrospective and perspectives[J].Advances in Colloid and Interface Science,2015,222:398-412.
[12] SCHELUDKO A.Advances in the investigation of thin films[J].Pure and Applied Chemistry,1965,10:323-336.
[13] WANG L.Drainage and rupture of thin foam films in the presence of ionic and non-ionic surfactants[J].International Journal of Mineral Processing,2012,102-103:58-68.
[14] SCHELUDKO A.Thin liquid films[J].Advances in Colloid and Interface Science,1967,1(4):391-464.
[15] PAN L,YOON R H.Hydrophobic forces in the wetting films of water formed on xanthate-coated gold surfaces[J].Faraday Discussions,2010,146:325-340.
[16] PAN L,JUNG S,YOON RH.Effect of hydrophobicity on the stability of the wetting films of water formed on gold surfaces[J].Journal of Colloid and Interface Science,2011,361(1):321-330.
[17] ISRAELACHVILI J,TABOR D.Measurement of van der Waals dispersion forces in the range 1.4 to 130 nm[J].Proceedings of the Royal Society of London,1972,331(1584):19-38.
[18] ISRAELACHVILI J,MIN Y,AKBULUT M,et al.Recent advances in the surface forces apparatus (SFA) technique[J].Reports on Progress in Physics,2010,73(3):036601.
[19] ISRAELACHVILI J,ADAMS G.Direct measurement of long range forces between two mica surfaces in aqueous KNO3 solutions[J].Nature,1976,262:774-776.
[20] HORN R G,BACHMANN D,CONNOR J,et al.The effect of surface and hydrodynamic forces on the shape of a fluid drop approaching a solid surface[J].Journal of Physics:Condensed Matter,1996,8(47):9483.
[21] CONNOR J,HORN R G.The influence of surface forces on thin film drainage between a fluid drop and a flat solid[J].Faraday Discussions,2003,123:193-206.
[22] PUSHKAROVA RA,HORN R G.Bubble-solid interactions in water and electrolyte solutions[J].Langmuir,2008,24(16):8726-8734.
[23] MANICA R,CONNOR J,CLASOHM L Y,et al.Transient responses of a wetting film to mechanical and electrical perturbations[J].Langmuir,2008,24(4):1381-1390.
[24] CONNOR J N,HORN R G.Measurement of aqueous film thickness between charged mercury and mica surfaces:A direct experimental probe of the Poisson-Boltzmann distribution[J].Langmuir,2001,17(23):7194-7197.
[25] DEL Castillo L A,OHNISHI S,CARNIE S L,et al.Variation of local surface properties of an air bubble in water caused by its interaction with another surface[J].Langmuir,2016,32(30):7671-7682.
[26] PUSHKAROVA R A,HORN R G.Surface forces measured between an air bubble and a solid surface in water[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2005,261(1):147-152.
[27] BINNIG G,QUATE C F,GERBER C.Atomic force microscope[J].Physical Review Letters,1986,56(9):930-933.
[28] DUCKER W A,SENDEN T J,PASHLEY R M.Direct measurement of colloidal forces using an atomic force microscope[J].Nature,1991,353(6341):239-241.
[29] DUCKER W A,SENDEN T J,PASHLEY R M.Measurement of forces in liquids using a force microscope[J].Langmuir,1992,8(7):1831-1836.
[30] BUTT H J.Measuring electrostatic,van der Waals,and hydration forces in electrolyte solutions with an atomic force microscope[J].Biophysical Journal,1991,60(6):1438-1444.
[31] BUTT H J,CAPPELLA B,KAPPL M.Force measurements with the atomic force microscope:Technique,interpretation and applications[J].Surface Science Reports,2005,59(1-6):1-152.
[32] YIN X H,GUPTA V,DU H,et al.Surface charge and wetting characteristics of layered silicate minerals[J].Advances in Colloid and Interface Science,2012,179:43-50.
[33] ARITA T,KANDA Y,HAMABE H,et al.In situ morphology of cationic flocculants adsorbed on surfaces and their interaction forces investigated by atomic force microscopy[J].Langmuir,2003,19(17):6723-6729.
[34] MCNAMEE C E,BUTT H J,HIGASHITANI K,et al.Interaction of cationic hydrophobic surfactants at negatively charged surfaces investigated by atomic force microscopy[J].Langmuir,2009,25(19):11509-11515.
[35] FA K Q,NGUYEN A V,MILLER J D.Interaction of calcium dioleate collector colloids with calcite and fluorite surfaces as revealed by AFM force measurements and molecular dynamics simulation[J].International Journal of Mineral Processing,2006,81(3):166-177.
[36] FA K Q,TAO J A,NALASKOWSKI J,et al.Interaction forces between a calcium dioleate sphere and calcite/fluorite surfaces and their significance in flotation[J].Langmuir,2003,19(25):10523-10530.
[37] FA K Q,NGUYEN A V,MILLER J D.Hydrophobic attraction as revealed by AFM force measurements and molecular dynamics simulation[J].Journal of Physical Chemistry B,2005,109(27):13112-13118.
[38] XING Y,LI C,GUI X,et al.Interaction forces between paraffin/stearic acid and fresh/oxidized coal particles measured by atomic force microscopy[J].Energy & Fuels,2017,31(3):3305-3312.
[39] XING Y,GUI X,CAO Y.Effect of calcium ion on coal flotation in the presence of kaolinite clay[J].Energy & Fuels,2016,30(2):1517-1523.
[40] GUI X,XING Y,RONG G,et al.Interaction forces between coal and kaolinite particles measured by atomic force microscopy[J].Powder Technology,2016,301:349-355.
[41] HOLUSZKO M E,FRANZIDIS J P,MANLAPIG E V,et al.The effect of surface treatment and slime coatings on ZnS hydrophobicity[J].Minerals Engineering,2008,21(12-14):958-966.
[42] LONG J,XU Z H,MASLIYAH J H.Role of illite-illite interactions in oil sands processing[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2006,281(1-3):202-214.
[43] LIU J,XU Z,MASLIYAH J H.Role of fine clays in bitumen extraction from oil sands[J].AIChE Journal,2004,50(8):1917-1927.
[44] LIU J J,XU Z H,MASLIYAH J.Studies on bitumen-silica interaction in aqueous solutions by atomic force microscopy[J].Langmuir,2003,19(9):3911-3920.
[45] LIANG Y,HILALN,LANGSTON P,et al.Interaction forces between colloidal particles in liquid:Theory and experiment[J].Advances in Colloid and Interface Science,2007,134-135:151-166.
[46] NGUYEN A V,NALASKOWSKI J,MILLER J D.Bubble-particle interaction measured by atomic force microscopy[J].Journal of Chemical Engineering of Japan,2015,37(2):231-237.
[47] ALBIJANIC B,OZDEMIR O,HAMPTON M A,et al.Fundamental aspects of bubble-particle attachment mechanism in flotation separation[J].Minerals Engineering,2014,65:187-195.
[48] ASSEMI S,NGUYEN A V,MILLER J D.Direct measurement of particle-bubble interaction forces using atomic force microscopy[J].International Journal of Mineral Processing,2008,89(1-4):65-70.
[49] GILLIES G,KAPPL M,BUTT H J.Direct measurements of particle-bubble interactions[J].Advances in Colloid and Interface Science,2005,114:165-172.
[50] PREUSS M,BUTT H J.Direct measurement of particle-bubble interactions in aqueous electrolyte:Dependence on surfactant[J].Langmuir,1998,14(12):3164-3174.
[51] ISHIDA N.Direct measurement of hydrophobic particle-bubble interactions in aqueous solutions by atomic force microscopy:Effect of particle hydrophobicity[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2007,300(3):293-299.
[52] XING Y,XU X,GUI X,et al.Effect of kaolinite and montmorillonite on fine coal flotation[J].Fuel,2017,195:284-289.
[53] XING Y,XU M,GUI X,et al.The application of atomic force microscopy in mineral flotation[J].Advances in Colloid and Interface Science,2018,10.1016/j.cis.2018.01.004.
[54] DUCKER W A,XU Z,ISRAELACHVILI J N.Measurements of hydrophobic and DLVO forces in bubble-surface interactions in aqueous solutions[J].Langmuir,1994,10(9):3279-3289.
[55] BUTT H J.A technique for measuring the force between a colloidal particle in water and a bubble[J].Journal of Colloid and Interface Science,1994,166(1):109-117.
[56] PREUSS M,BUTT H J.Direct measurement of forces between particles and bubbles[J].International Journal of Mineral Processing,1999,56(1-4):99-115.
[57] NGUYEN A V,NALASKOWSKI J,MILLER J D.A study of bubble-particle interaction using atomic force microscopy[J].Minerals Engineering,2003,16(11):1173-1181.
[58] NGUYEN A V,EVANS G M,NALASKOWSKI J,et al.Hydrodynamic interaction between an air bubble and a particle:Atomic force microscopy measurements[J].Experimental Thermal and Fluid Science,2004,28(5):387-394.
[59] GILLIES G,PRESTIDGE C A,ATTARD P.Determination of the separation in colloid probe atomic force microscopy of deformable bodies[J].Langmuir,2001,17(25):7955-7956.
[60] GILLIES G,PRESTIDGE C A.Interaction forces,deformation and nano-rheology of emulsion droplets as determined by colloid probe AFM[J].Advances in Colloid and Interface Science,2004,108-109:197-205.
[61] TARAN E,HAMPTON M A,NGUYEN A V,et al.Anomalous time effect on particle-bubble interactions studied by atomic force microscopy[J].Langmuir,2009,25(5):2797-2803.
[62] CHAN D,DAGASTINE R,WHITE L.Forces between a rigid probe particle and a liquid interface:I.The repulsive case[J].Journal of Colloid and Interface Science,2001,236(1):141-154.
[63] DAGASTINE R,WHITE L.Forces between a rigid probe particle and a liquid interface:II.The general case[J].Journal of Colloid and Interface Science,2002,247(2):310-320.
[64] DAGASTINE R,PRIEVE D C,WHITE L.Forces between a rigid probe particle and a liquid interface:III.Extraction of the planar half-space interaction energy E(D)[J].Journal of Colloid and Interface Science,2004,269(1):84-96.
[65] ENGLERT A H,REN S,MASLIYAH J,et al.Interaction forces between a deformable air bubble and a spherical particle of tuneable hydrophobicity and surface charge in aqueous solutions[J].Journal of Colloid and Interface Science,2012,379:121-129.
[66] CHAN D Y,KLASEBOER E,MANICA R.Theory of non-equilibrium force measurements involving deformable drops and bubbles[J].Advances in Colloid and Interface Science,2011,165(2):70-90.
[67] MANOR O,VAKARELSKI I U,STEVENS G W,et al.Dynamic forces between bubbles and surfaces and hydrodynamic boundary conditions[J].Langmuir,2008,24(20):11533-11543.
[68] MANOR O,VAKARELSKI I U,TANG X S,et al.Hydrodynamic boundary conditions and dynamic forces between bubbles and surfaces[J].Physical Review Letters,2008,101(2):024501.
[69] DAGASTINE R,MANICA R,CARNIE S L,et al.Dynamic forces between two deformable oil droplets in water[J].Science,2006,313(5784):210-213.
[70] VAKARELSKI I U,LI E Q,THORODDSEN S T.Soft colloidal probes for AFM force measurements between water droplets in oil[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2014,462:259-263.
[71] VAKARELSKI I U,MANICA R,TANG X,et al.Dynamic interactions between microbubbles in water[J].Proceedings of the National Academy of Sciences,2010,107(25):11177-11182.
[72] TABOR RF,WU C,LOCKIE H,et al.Homo-and hetero-interactions between air bubbles and oil droplets measured by atomic force microscopy[J].Soft Matter,2011,7(19):8977-8983.
[73] SHI C,CHAN D Y C,LIU Q X,et al.Probing the hydrophobic interaction between air bubbles and partially hydrophobic surfaces using atomic force microscopy[J].Journal of Physical Chemistry C,2014,118(43):25000-25008.
[74] CUI X,SHI C,ZHANG S,et al.Probing effect of salinity and pH on surface interactions between air bubbles and hydrophobic solids:Implications on colloidal assembly at air/water interface[J].Chemistry-An Asian Journal,2017,12(13):1568-1577.
[75] XIE L,SHI C,WANG J Y,et al.Probing the interaction between air bubble and sphalerite mineral surface using atomic force microscope[J].Langmuir,2015,31(8):2438-2446.
[76] XIE L,WANG J,YUAN D,et al.Interaction mechanisms between air bubble and molybdenite surface:Impact of solution salinity and polymer adsorption[J].Langmuir,2017,33(9):2353-2361.
[77] TABOR R F,LOCKIE H,MAIR D,et al.Combined AFM-confocal microscopy of oil droplets:Absolute separations and forces in nanofilms[J].Journal of Physical Chemistry Letters,2011,2(9):961-965.
[78] PRIEVE D C.Measurement of colloidal forces with TIRM[J].Advances in Colloid and Interface Science,1999,82(1-3):93-125.
[79] CLARK S C,WALZ J Y,DUCKER W A.Atomic force microscopy colloid-probe measurements with explicit measurement of particle-solid separation[J].Langmuir,2004,20(18):7616-7622.
[80] CONTRERAS-NARANJO J C,UGAZ V M.A nanometre-scale resolution interference-based probe of interfacial phenomena between microscopic objects and surfaces[J].Nature Communications,2013,4(5):1919.
[81] PAN L,YOON R H.Measurement of hydrophobic forces in thin liquid films of water between bubbles and xanthate-treated gold surfaces[J].Minerals Engineering,2016,98:240-250.
[82] SHAHALAMI M,WANG L,WU C,et al.Measurement and modeling on hydrodynamic forces and deformation of an air bubble approaching a solid sphere in liquids[J].Advances in Colloid and Interface Science,2015,217:31-42.
[83] IVANOVA N O,XU Z,LIU Q,et al.Surface forces in unconventional oil processing[J].Current Opinion in Colloid & Interface Science,2017,27:63-73.
[84] WANG L,SHARP D,MASLIYAH J,et al.Measurement of interactions between solid particles,liquid droplets,and/or gas bubbles in a liquid using an integrated thin film drainage apparatus[J].Langmuir,2013,29(11):3594-3603.
[85] SHI C,CUI X,ZHANG X R,et al.Interaction between air bubbles and superhydrophobic surfaces in aqueous solutions[J].Langmuir,2015,31(26):7317-7327.
[86] ZHANG X,TCHOUKOV P,MANICA R,et al.Simultaneous measurement of dynamic force and spatial thin film thickness between deformable and solid surfaces by integrated thin liquid film force apparatus[J].Soft Matter,2016,12(44):9105-9114.
[87] ZHANG X,MANICA R,TCHOUKOV P,et al.Effect of approach velocity on thin liquid film drainage between an air bubble and a flat solid surface[J].Journal of Physical Chemistry C,2017,121(10):5573-5584.
[88] ZHANG X,MANICA R,TANG Y,et al.Probing boundary conditions at hydrophobic solid-water interfaces by dynamic film drainage measurement[J].Langmuir,2018,34(40):12025-12035.
