准东煤田是中国目前发现的最大整装煤田,其预测储量达3 900亿t[1],准东煤主要用于煤电及煤化工行业,是国家“疆电外送”基地,新疆经济的发展必然需要准东煤的大规模开发利用。准东煤优点在于着火温度低、燃尽率高,是优良的动力用煤,虽然准东煤原煤中碱/碱土金属(AAEM)含量不高,但由于其灰分含量低,准东煤灰中Na2O,CaO含量较高[2]。实际燃用准东煤过程中,无论是煤粉锅炉,还是循环流化床锅炉,在炉膛至空气预热器均出现了不同程度的结渣、沾污问题[3-5]。个别燃用氯含量较高的准东煤锅炉发生了高温腐蚀。
煤灰组分复杂,一般可分为酸性氧化物(SiO2,Al2O3,TiO2 等)和碱性氧化物(CaO,MgO,Na2O,K2O,Fe2O3等)。准东煤灰中Na2O(最高达15.92%),CaO/Fe2O3(最高达55.88%/28.64%)等碱性氧化物含量较高,结渣倾向严重。为应对燃用准东煤产生的结渣、沾污问题,学者们对准东煤热转化过程中AAEM释放及迁移规律,灰熔融、结渣及沾污特性进行了大量研究[6-9],据此为在役燃用准东煤锅炉优化运行及新建锅炉选型提供指导,结渣、沾污防控技术主要包括应用液态排渣炉、降低烟气流速以减少迎风面沾污、增大炉膛容积降低炉膛出口烟气温度、掺烧高岭土等。目前运行效果较好的煤粉炉,准东煤掺烧比例最高可达80%~90%[10-11]。然而以上方法的应用仅可改善锅炉结渣、沾污问题,并不能从根本上解决准东煤安全、高效、洁净利用的难题。
随着锅炉主蒸汽参数的不断提高,受热面布置、烟温等的改变可能引起更严重的结渣、沾污问题。笔者总结了目前准东煤热转化过程中AAEM形态演化及矿物转化特性,煤灰熔融特性,结渣、沾污特性。
1 碱/碱土金属的赋存形态及迁移规律
高碱燃料中AAEM在热转化过程中的释放及在受热面上的冷凝是沾污形成的关键步骤。研究AAEM在准东煤中的赋存形态和迁移规律对解决燃用准东煤锅炉结渣、沾污问题具有重要意义。
1.1 煤中AAEM测定方法及赋存形态
1.1.1 煤中AAEM测定方法
准东煤中碱金属以钠为主,碱土金属以钙为主。由于煤中的有机物会影响矿物测试结果的准确性,煤中钠和钙含量的测试通常采用高温灰样(基于《煤灰成分分析方法》,GB/T 1574—2007),并依据煤灰组分分析结果建立了包括碱酸比、当量Na2O在内的一系列结渣、沾污倾向性评价指标。部分学者将煤灰中AAEM氧化物含量折算为煤中AAEM含量,但由于灰化温度的升高会促进煤中AAEM的释放,所获得煤中AAEM含量具有较大偏差。LI等[12]和QUYN等[13]发现当热解温度为300~400 ℃时,约有10%的AAEM从煤中释放出来。Na的释放速率与煤中的NaCl含量呈正相关。还发现随着灰化温度的增加,煤灰中的Na逐渐从水溶性Na变为不可溶态Na[14-15]。与Na不同,杨燕梅等[16-17]和付子文等[18]认为灰化温度的增加会促进碱金属的挥发,而碱土金属的释放基本不受灰化温度的影响。
815 ℃制取的煤灰中AAEM含量不能准确反映煤中AAEM的含量。因此,部分学者提出了低温灰化法、逐级萃取法(浸渍法)、微波消解、氧弹燃烧法等高碱煤AAEM含量测试方法。一般认为逐级萃取法、氧弹法测试过程中无AAEM的释放,最为精确。逐级萃取法可获得煤中不同形态AAEM所占比例,因而获得了广泛应用。
1.1.2 煤中AAEM赋存形态
AAEM在煤中的赋存形态是影响其热转化过程中释放特性的重要因素。一般认为煤中的AAEM可以分为有机形式和无机形式[19]。有机形式主要包括羧酸盐或AAEM以配位形式存在于煤中含氧或者含氮官能团中。无机形式主要为含AAEM的无机盐、水合离子及晶体等。
对煤中AAEM赋存形式的研究主要采用逐级萃取法。根据AAEM在不同提取液中的溶解特性,可将煤中AAEM分为水溶态、醋酸铵溶态、酸溶态及不可溶态。BENSON和HOLM[20]首次提出采用乙酸铵(CH3COONH4)和稀盐酸(HCl)作为提取液分析煤中Na和Ca的赋存形态。采用逐级萃取法对准东煤中AAEM的赋存形态进行分析,结果表明,准东煤中Na以水溶态和醋酸铵溶态为主(水溶态占比 40%~80%),酸溶态和不溶态Na含量较低;Ca则主要为醋酸铵溶态、酸溶态,约占90%左右,不可溶态Ca含量低于10%[21]。
曾宪鹏等[22]对马弗炉制备的低温灰(350 ℃)进行逐级提取及分析,发现准东煤中Na以水溶态为主,Ca和Mg主要为乙酸铵溶态和盐酸溶态。
1.2 煤中AAEM的迁移规律
煤热转化过程中,含AAEM的矿物随着温度的升高逐渐分解,部分AAEM从煤中析出释放到气相中。AAEM的释放主要与其在煤中赋存形态、温度、升温速率、气氛等因素相关。
LI[23]利用金属丝网反应器分析讨论了醋酸铵溶态AAEM的释放特性,醋酸铵溶态AAEM的释放主要受温度的影响,加热速率对其释放影响较小。KOSMINSKI等[24]的热解与气化实验表明,相同条件下,NaCl比CH3COONa更容易释放,且气氛对添加NaCl样品Na的释放影响不大,对添加CH3COONa样品Na的释放影响较大。
煤中Na的释放分为燃烧初期和燃烧后期[19],燃烧初期煤中钠并未从煤中析出,只是进行不同赋存形态间的相互转化;燃烧后期钠从煤中析出并与烟气发生反应形成新的含钠化合物,主要包括钠的(铝)硅酸盐、氯化钠、硫酸钠和氢氧化钠4种[25-28]。准东煤燃烧过程中,Na在400~600 ℃快速释放,1 000 ℃以内Na的释放主要以水溶态Na和有机Na为主[29]。当温度低于500 ℃,少量有机Na释放,当温度高于600 ℃,水溶态Na开始析出。在热转化过程中,水溶态Na逐渐向酸溶态Na转变,可溶态Na逐渐向不可溶态Na转变。LI等[30]发现在300~600 ℃,温度不会影响Ca的释放。在600~900 ℃,Ca的释放速率随温度的升高而增加。魏砾宏等[31]通过分析Na形态/含量、Cl含量、灰中矿物质含量、燃烧温度对Na挥发量的影响,认为温度是影响Na挥发量的主要因素,Si/Al等矿物质可以通过化学反应抑制Na的挥发,Ca/Mg等则通过消耗Si/Al间接促进Na的释放,Cl含量与Na挥发量之间并未有显著联系。
限于技术条件,当前的在线检测技术[32-34]如等离子体激发原子共振线性光谱法、准分子激光诱导荧光碎片、激光诱导击穿光谱法等仅能应用于气象AAEM定量检测,无法区分AAEM具体形态。BLASING和MULLER[35]采用分子束质谱测试了气化和燃烧过程中Na,Cl,S 等元素的释放规律,实验发现,快速加热条件下,HCl,NaCl,NaOH 的释放发生在挥发分释放阶段。Na 的主要释放形式为 NaCl 和 NaOH。
2 煤灰熔融特性
2.1 灰熔融特性研究方法
煤灰的熔融或烧结是结渣、沾污发展的重要步骤。煤灰的熔融特性被用作评价煤种的结渣、沾污倾向。
学者们通过膨胀/收缩试验[36-38],烧结强度试验[39-40]及一系列经验指标研究煤灰熔融特性。煤灰的熔融动态特性可以通过熔融曲线即熔融灰分的比例与温度的关系来描述[41]。由于前者实验时间较长,灰熔融特性一般采用灰熔融特征温度(《煤灰熔融特性的测定方法》,GB/T 219—2008)进行表征[42]。采用灰锥法,使用电炉进行加热,记录灰锥不同变形程度对应的4个特征温度,即为灰熔融特征温度,包括:变形温度(TD),软化温度(TS),半球温度(TH)和流动温度(TF)。目前用于灰熔融特征温度测试的样品制备方法依赖于手工制成的圆锥形或圆柱状灰,但由于手工制灰锥导致可重复性差,以及结果的不确定性和主观性[43]。
部分学者通过混烧准东煤与其他煤种改变煤灰熔融特性,通过混烧增加煤灰中的酸性氧化物可提高煤灰的烧结温度,但由于灰熔融过程中可能形成低熔点共晶化合物,导致灰熔融特征温度低于准东煤灰。混烧对煤灰熔融特性的影响尚未得到充分研究。此外,煤灰熔融特性研究一般采用实验室制灰,灰化温度会影响煤灰的化学和矿物组成[44]。准东煤灰中的Na随着灰化温度的升高大量释放[45]。在表征灰熔融特性时不能忽视灰化温度的影响。
2.2 灰熔融特征温度预测模型
准东煤煤灰熔融过程中,通过复杂的物理化学反应生成大量富含Ca/Mg/Fe的助融矿物(黄长石,橄榄石等),Na也与SiO2,Al2O3等反应生成具有助融效果的霞石、偏铝酸钠等。煤灰的灰熔融特性与SiO2,Al2O3,CaO,MgO,NaO,FeO含量密切相关。
学者们基于煤灰组分建立了灰熔融特征问题的预测模型。常用的灰熔融特征温度预测模型包括基于煤灰组分建立了煤灰流动温度预测模型[46-47](式(1),(2))及通过Factsage计算获得流动温度与液相线温度经验关系式。
TF=0.903×(85%movement)+158
85%movement=1 340lg Al2O3-251lg Fe2O3-
106lg CaO-172
(1)
TF=1 463.055-376.865x+181.35x2-
33.485x3+2.735 5x4-0.082 5x5
![]()
(2)
许洁等[48]则基于Factsage软件以煤灰组分为输入条件计算获得煤灰的液相线温度Tliquidus,通过实验拟合获得煤灰流动温度与其液相线温度间的经验关联式:
TF=aFT+bFTTliquidus
(3)
其中,aFT,bFT为通过数据拟合获得的相关系数。杨燕梅[49]通过灰熔融特征温度测试和Factsage计算对准东煤灰熔融特性进行研究,通过对比实验获得的灰熔融特征温度和不同模型计算结果,发现在预测准东煤灰熔融特征温度时不可忽略碱金属的影响,许洁等提出的基于煤灰液相线温度的流动温度预测公式与实验结果吻合较好,并给出了经验系数(aFT=0.615 62,bFT=411)。
杨燕梅对比了多种准东煤煤灰的灰熔融特征温度及预测模型获得的煤灰流动温度,见表1。
对比表1中的实验测量值及模型预测值(分别由式(1),(2),(3)计算获得),基于液相线温度和流动温度的预测模型流动温度偏差小于50 ℃,预测效果由于基于煤灰组分的预测结果。
3 煤灰沉积特性
准东煤由于煤灰中AAEM含量较高导致产生严重的沾污结渣问题。灰沉积包括结渣(主要发生在辐射受热面上)、沾污(主要发生在对流受热面)。研究灰沉积特性主要有两种研究方法。一种是对真实锅炉中收集的灰沉积样品进行形态和组分分析[50-51]。另一种是使用灰采样管来收集实际锅炉[52-53]或粉煤燃烧试验台(如落管炉,一维炉等)[54-55]中的灰样。
表1 煤灰流动温度对比[49]
Table 1 Comparison of FT of different coal ash[49]
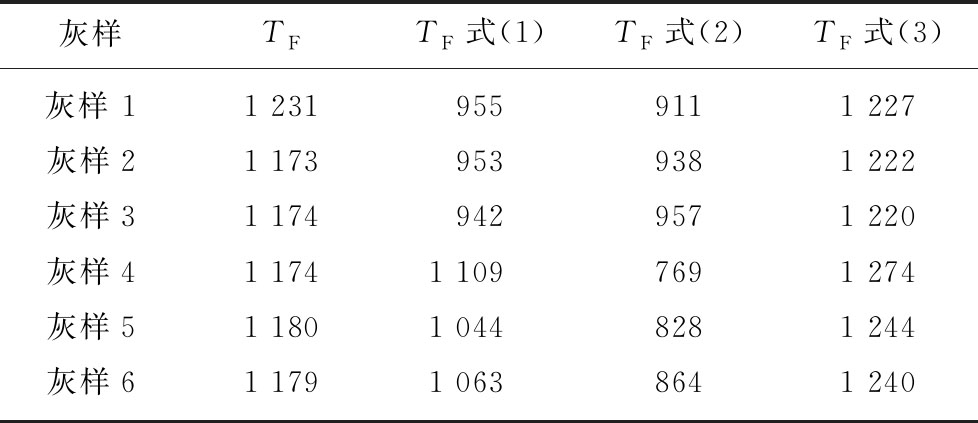
灰样TFTF式(1)TF式(2)TF式(3)灰样 1 1 2319559111 227灰样21 1739539381 222灰样31 1749429571 220灰样41 1741 1097691 274灰样51 1801 0448281 244灰样61 1791 0638641 240
3.1 结 渣
结渣由熔融或部分熔融的灰颗粒碰撞并冷凝在温度较低的水冷壁或主要辐射受热面上形成。实际燃用准东煤过程中出现了严重结渣[56],如图1所示。
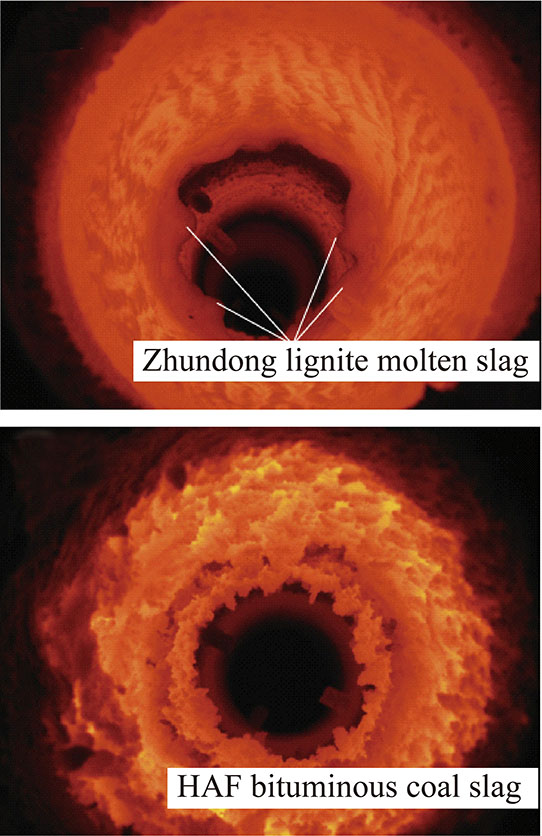
图1 燃烧器入口结渣情况[51]
Fig.1 Coal slagging near burner inlet[51]
根据VORRES[57]提出的“离子势”(即离子化合价与离子半径之比)概念,酸性组分阳离子的离子势高,易与氧结合形成多聚物,能提高熔融温度,碱性组分能终止多聚物的生成,降低熔融温度。VASSILEV等[58]发现煤灰中硫酸盐,硅酸盐和氧化物,如硬石膏,钾长石,硅酸钙和赤铁矿的比例增加可提高灰熔融特征温度。偏高岭土,莫来石则具有助融效果。对于准东煤灰,由于碱金属和碱土金属含量较高,存在较多助熔矿物(石英,硬石膏和岩盐)从而降低了烧结和熔融温度[58-59],促进锅炉结渣。
熔融的煤灰是结渣的主要组分,其元素组成主要包括Si/Al/Ca[56]。煤灰组分对结渣具有较大影响。准东煤煤灰中Na含量较高,且易与SiO2,Al2O3等反应生成具有助融效果的霞石、偏铝酸钠等促进结渣。Fe可与Si/Al/Ca反应形成低熔点共晶盐,这将促进结渣。准东煤煤灰中富含Na,Ca,Mg及Fe等矿物,极大促进了锅炉结渣。唐诗等[60]综合TGA-DSC及XRD数据认为720~910 ℃碱方解石和硬石膏的快速分解生成的大量CaO与Si/Mg等反应生成低熔点共晶化合物,从而促进结渣;硅酸钠是引起低温渣聚团的主要原因。
除了煤灰组分外,炉膛运行参数、燃烧气氛等同样会影响结渣。炉膛温度升高会促进硅铝酸钠等低熔点物质的生成,但同时会促进AAEM的释放,减少低熔点矿物的生成。温度越高,结渣越严重。在还原性气氛下,含Fe2+的熔融颗粒能增加灰颗粒黏性,使其更易于沉积和捕获灰颗粒[51,61-62],WEE等[61]研究了一次空气对实际锅炉结渣的影响,结果表明,氧化气氛可以减轻结渣。
3.2 沾 污
沾污指灰颗粒在对流受热面上的沉积。对于具有较强沾污性的准东煤,沾污灰样具有较强黏性且会不断捕捉烟气中细微灰颗粒加剧沾污。
大量学者对沾污机理进行了深入研究,如图2所示[63-67]。一般认为受热面沾污具有分层结构,通常包括内白层、烧结内层和外部烧结层[68-70]。针对燃用准东煤锅炉现场沉积样品和燃烧平台沉积样品均观察到了分层结构。

图2 受热面灰沉积机理[54](修改自文献[55-58])
Fig.2 Ash deposition mechanisms on a heat transfer tube[54](modified from[55-58])
燃用准东煤时,Na,Ca,Si,Cl,S 等元素在高温火焰区易从煤中释放并以NaCl,CaO,SiO2,CaCl2等形式存在与烟气中。部分Na蒸气被煤灰捕捉,通过物理吸附或化学反应的方式固定在灰中,部分则会冷凝在受热面壁面上,并与金属管壁以及烟气中其他矿物质相互作用形成沾污内层。 沾污内层的组成和粒度分布与外层的组成和粒度分布明显不同,主要通过挥发性矿物的气相扩散冷凝和细颗粒的热迁移形成,细颗粒通过范德华力和静电力附着在加热表面上。沾污内层主要由富含黏性成分的细颗粒(<2 μm)组成[71]。Na,Ca和S是构成沾污内层的主要元素。SONG等[72]发现CaSO4-Na2SO4在沾污中起重要作用。史航等[73]发现CaSO4/Na2SO4/Ca-Si-Al是沾污样品的主要组分,沾污内层以Na2SO4为主。沾污内层且AAEM含量高,黏性大,可将积灰固定在受热面,且热阻大,会影响受热面的传热效率,导致沾污表面温度升高并形成熔融或半熔融基体,从而捕捉大量惯性撞击到沾污表面的灰颗粒,促进积灰的生长。
史航等对实际燃用准东煤锅炉烟气沿程灰沉积样品进行分析,发现AAEM和S在高温再热器沉积样品内层中发生了富集,CaSO4,Na2SO4含量远高于其他沉积样品。关于气相AAEM的硫化发生是均相反应[74]还是异相反应[75]目前尚无定论。
4 准东煤结渣、沾污主要影响因素及防控方法
根据前述章节,分析准东煤结渣、沾污的主要影响因素及控制方法。
4.1 准东煤结渣主要影响因素及防控方法
炉膛结渣是一个复杂的物理化学过程。煤灰组分、炉膛参数、气氛等均对结渣具有重大影响。综合前述内容,准东煤中Na主要以水溶态和醋酸铵溶态,在炉膛煤粉高温燃烧区域Na会快速从煤中释放,部分Na与SiO2,Al2O3等反应生成具有助融效果的霞石、偏铝酸钠碰撞到水冷壁后形成熔融状的结渣底层,并不断捕捉灰颗粒,促进结渣过程。目前,针对炉膛结渣的防控方法主要包括:
(1)添加高岭土等酸性矿物[76]。高岭土、方解石、Mg、Fe等添加剂可以抑制AAEM的释放,并吸附烟气中的Na。添加剂通过与AAEM反应减少低熔点共晶化合物的生成,提高煤灰灰熔融特征温度。
(2)掺烧低碱煤[77]。掺烧低碱煤可以改变煤灰理化特性,减少准东煤热转化过程中挥发性物质的释放。同时,可以通过调整S/Cl比例减轻锅炉腐蚀。
(3)水洗[78]。准东煤中Na主要以水溶态和醋酸铵溶态为主,且对结渣具有促进作用。水洗可有效降低煤中Na,S等元素的含量从而有效减轻结渣。
(4)锅炉运行参数调整[79]。高温烟气中碱金属的在水冷壁上的凝结是结渣底层形成的关键因素。控制水冷壁近壁面区域的烟气温度,可减小炉膛结渣的可能性。
4.2 准东煤沾污主要影响因素及防控方法
锅炉受热面沾污涉及挥发性矿物的释放、冷凝和矿物间的化学反应。沾污具有显著的分层结构,沾污内层主要由AAEM的气相扩散冷凝及细微颗粒热迁移形成,外层的形成主要由惯性撞击主导。根据沉积样品分析,AAEM的硫酸盐的形成及在受热面上的凝结是沾污形成的关键因素。
由于准东煤结渣、沾污与其煤灰中高含量的AAEM密切相关,因此二者的防控方法均包括添加高岭土等矿物、掺烧低碱煤、进行水洗等预处理。此外,针对炉膛结渣的防控方法还包括:
(1)增加吹灰时间和吹灰频率。由于准东煤沾污样品主要由富含AAEM矿物构成,沾污样品结构致密,机械吹灰的方法很难达到理想效果。蒸汽吹灰对准东煤沾污清除效果目前尚未明确。
(2)锅炉运行参数调整。对流受热面沾污是一个复杂的传热、传质过程,通过拉大易沾污区受热面节距,降低烟气流速,降低炉膛出口烟气温度可降低灰颗粒与受热面撞击概率,减小灰颗粒黏性,缓解受热面沾污情况。
(3)采用热解或气化技术。利用热解和气化技术提前移除准东煤中的易挥发性矿物并抑制AAEM在高温下的析出是实现准东煤安全、高效、洁净利用的可能手段。具体效果仍有待研究。
5 分析与展望
准东地区低碱煤储量不到高碱煤储量1%,按照现役机组准东煤掺烧比例(最高可达80%~90%[10-11]),准东低碱煤无法满足准东煤大规模开发利用需求,全烧准东煤势在必行。目前,通过锅炉设计、掺烧、参数调整等仅能减轻准东煤结渣、沾污等问题,尚未有从根本上解决全烧准东煤的成熟技术。
目前,针对准东煤中AAEM的赋存、释放、迁移及灰的结渣、沾污特性已有大量研究。但仍有一些不足有待进一步研究:由于实验样品和条件的差异,AAEM在煤燃烧过程中的释放、转化特性及其对灰熔融特性的影响尚未有一致结论;对于AAEM释放多采用热力学平衡计算,相应的动力学模型缺乏深入研究;由于燃烧试验台实验工况与实际锅炉烟气组分,热参数等存在一定差异,燃烧试验台获得的结渣、积灰特性能否反映真实锅炉情况有待验证;实际锅炉沉积样品分析面临锅炉工况调整困难、可重复性差等问题,需对锅炉实际结渣污特性进行深入研究。
综上,可采用配制煤灰研究灰化温度、气氛等对煤热转化中AAEM的释放和转化特性,以此降低实验样品的影响;锅炉结渣、沾污是动态长期过程,AAEM盐的硫酸盐化对AAEM蒸气在受热面壁面的冷凝沉积具有重要影响,建立AAEM在煤热转化过程中的释放、矿物转化动力学模型是重要方向;需综合燃烧试验台和实际锅炉实验沉积样品分析结果,研究温度、气氛、煤组分等对准东煤结渣、沾污趋势变化规律的影响,为结渣、沾污防控提供依据。
6 结 语
准东煤作为优良的动力用煤,主要用于煤电和煤化工行业,其开发利用对于国家的能源保障具有重要意义。但准东煤灰中碱/碱土金属含量高,在燃烧过程中易挥发进入气相并冷凝在受热面上从而强化锅炉的沾污、结渣过程。目前,尚无解决准东煤及其类似煤种燃烧过程中出现的严重结渣、沾污问题的可靠技术方案,为实现准东煤的安全、高效、洁净利用,仍需更加深入的研究煤灰结渣、沾污机理:加强灰化温度、气氛等对煤热转化中AAEM的释放和转化特性影响的系统研究;建立AAEM在煤热转化过程中的释放、矿物转化动力学模型;综合燃烧试验台和实际锅炉实验沉积样品分析结果,研究温度、气氛、煤组分等对准东煤结渣、沾污趋势变化规律的影响,为结渣、沾污防控提供依据。
[1] XU L,LIU H,FANG H,et al.Effects of various inorganic sodium salts present in Zhundong coal on the char characteristics[J].Fuel,2017,203:120-127.
[2] 赵勇纲,史航,吴玉新,等.高碱煤中碱金属及氯元素析出特性[J].洁净煤技术,2018,24(5):33-37.
ZHAO Yonggang,SHI Hang,WU Yuxin,et al.Release characteristics of alkali metal and Cl species in high alkali coal[J].Clean Coal Technology,2018,24,24(5):33-37.
[3] LI G Y,WANG G C A,WANG P Q,et al.Ash deposition and alkali metal migration during Zhundong high-alkali coal gasification[J].Energy Procedia,2017,105:1350-1355.
[4] WANG X B,XU Z X,WEI B,et al.The ash deposition mechanism in boilers burning Zhundong coal with high contents of sodium and calcium:A study from ash evaporating to condensing[J].Applied Thermal Engineering,2015,80:150-159.
[5] WANG C,JIN X,WANG Y,et al.Release and transformation of sodium during pyrolysis of Zhundong coals[J].Energy & Fuels,2015,29(1):78-85.
[6] ZHU C,QU S J,ZHANG J,et al.Distribution,occurrence and leaching dynamic behavior of sodium in Zhundong coal[J].Fuel,2017,190:189-197.
[7] DU Y B,WANG C A,LV Q,et al.Influence of sodium on deactivation and regeneration of SCR catalyst during utilization of Zhundong coals[J].Asia-Pacific Journal of Chemical Engineering,2016,11(6):973-980.
[8] BRYERS R W.Fireside slagging,fouling,and high-temperature corrosion of heat-transfer surface due to impurities in steam-raising fuels[J].Progress in Energy and Combustion Science,1996,22(1):29-120.
[9] LI J B,ZHU M M,ZHANG Z Z,et al.The mineralogy,morphology and sintering characteristics of ash deposits on a probe at different temperatures during combustion of blends of Zhundong lignite and a bituminous coal in a drop tube furnace[J].Fuel Processing Technology,2016,149:176-186.
[10] 李路明.燃烧新疆准东煤350 MW超临界锅炉设计应用[J].电站系统工程,2014,2:39-41.
LI Luming.Design and application of 350 MW supercritical boiler burning zhundong coal[J].Power System Engineering,2014,2:39-41.
[11] 李江浩.电站锅炉高比例掺烧准东煤的运行与调整[J].锅炉技术,2016,2:1-3.
LI Jianghao.Operation and adjustment of high percentage blending zhundong coal in utility plant[J].Boiler Technology,2016,2:1-3.
[12] LI C Z.Importance of volatile-char interactions during the pyrolysis and gasification of low rank fuels-A review[J].Fuel,2013,112:609-623.
[13] QUYN D M,WU H,LI C Z.Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal.Part I.Volatilisation of Na and Cl from a set of NaCl-loaded samples[J].Fuel,2002,81(2):143-149.
[14] LI G Y,WANG C A,Yan Y,et al.Release and transformation of sodium during combustion of Zhundong coals[J].Journal of the Energy Institute,2016,89(1):48-56.
[15] XU L,LIU H,ZHAO D,et al.Transformation mechanism of sodium during pyrolysis of Zhundong coal[J].Fuel,2018,233:29-36.
[16] 杨燕梅,张海,吴玉新,等.不同灰化温度下准东煤碱/碱土金属析出特性[J].燃烧科学与技术,2015,21(4):297-300.
YANG Yanmei,ZHANG Hai,WU Yuxin,et al.Release of alkali /alkaline earth metal species in Zhundong coal at different ashing temperatures[J].Journal of Combustion Science and Technology,2015,21(4):297-300.
[17] 杨燕梅,杨欣华,刘青,等.灰化温度对准东煤灰组分分析的影响[J].煤炭学报,2016,41(10):2441-2447.
YANG Yanmei,YANG Xinhua,LIU Qing,et al.Effect of ashing temperature on analysis of Zhundong coal ash[J].Journal of China Coal Society,2016,41(10):2441-2447.
[18] 付子文,王长安,车得福,等.成灰温度对准东煤灰理化特性影响的实验研究[J].工程热物理学报,2014,35(3):609-613.
FU Ziwen,WANG Chang’an,CHE Defu,et al.Experimental study on the effect of ashing temperature on physicochemical properties of Zhundong coal ashes[J].Journal of Engineering Thermophysics,2014,35(3):609-613.
[19] 李勇,肖军.燃煤过程中碱金属赋存迁移规律及相关研究进展[J].洁净煤技术,2005,11(1):39-44.
LI Yong,XIAO Jun.The occurrence and migration mechanism of alkali metal during coal-fired process and research progress[J].Clean Coal Technology,2005,11(1):39-44.
[20] BENSON S A,HOLM P L.Comparison of inorganics in three low-rank coals[J].Industrial & Engineering Chemistry Product Research and Development,1983,24(1):145-149.
[21] YANG Y,WU Y,ZHANG H,et al.Improved sequential extraction method for determination of alkali and alkaline earth metals in Zhundong coals[J].Fuel,2016,181:951-957.
[22] 曾宪鹏,于敦喜,于戈,等.准东煤燃烧中不同形态无机元素向颗粒物的转化行为[J].煤炭学报,2019,44(2):588-595.
ZENG Xianpeng,YU Dunxi,YU Ge,et al.Transformation of inorganic elements in different forms into ash particles during Zhundong coal combustion[J].Journal of China Coal Society,2019,44(2):588-595.
[23] LI C Z.Importance of volatile-char interactions during the pyrolysis and gasification of low rank fuels-A review[J].Fuel,2013,112(3):609-623.
[24] KOSMINSKI A,ROSS D P,AGNEW J B.Transformations of sodium during gasification of low-rank coal[J].Fuel Processing Technology,2006,87(11):943-952.
[25] 陶玉洁,张彦威,周俊虎,等.准东煤在燃烧过程中的矿物演变过程及Na、Ca释放规律[J].中国电机工程学报,2015(5):1169-1175.
TAO Yujie,ZHANG Yanwei,ZHOU Junhu,et al.Mineral conversion regularity and release behavior of Na,Ca During Zhundong Coal’s Combustion[J].Proceedings of the CSEE,2015(5):1169-1175.
[26] RAASK E.Mineral impurities in coal combustion,behavior,problems,and remedial measures[M].New York:Hemisphere Publishing Corporation,1985.
[27] MANZOORI A R,Agarwal P K.The fate of organically bound inorganic elements and sodium chloride during fluidized bed combustion high sulphur low rank coals[J].Fuel,1992,71(5):513-522.
[28] 王智化,李谦,刘敬,等.准东煤中碱金属的赋存形态及其在热解过程中的迁移规律[J].中国电机工程学报,2014,34(S1):130-135.
WANG Zhihua,LI Qian,LIU Jing,et al.Occurrence of alkali metals in zhundong coal and its migration during pyrolysis process[J].Proceedings of the CSEE,2014,34(S1):130-135.
[29] 刘敬,王智化,项飞鹏,等.准东煤中碱金属的赋存形式及其在燃烧过程中的迁移规律实验研究[J].燃料化学学报,2014,42(3):316-322.
LIU Jing,WANG Zhihua,XIANG Feipeng,et al.Modes of occurrence and transformation of alkali metals in Zhundong coal during combustion[J].Journal of Fuel Chemistry and Technology,2014,42(3):316-322.
[30] LI C Z,SAHTE C,KERSHAW J R,et al.Fates and roles of alkali and alkaline earth metals during the pyrolysis of a Victorian brown coal[J].Fuel,2000,79(3):427-438.
[31] 魏砾宏,崔保崇,陈勇,等.高碱煤钠赋存形态及其燃烧过程中迁移转化的研究进展[J].燃料化学学报,2019,47(8):897-906.
WEI Lihong,CUI Baochong,CHEN Yong,et al.Occurrence of sodium in high alkali coal and its transformation during combustion[J].Journal of Fuel Chemistry and Technology,2019,47(8):897-906.
[32] HAYRINEN V,HERNBERG R,AHO M.Demonstration of plasma excited atomic resonance line spectroscopy for on-line measurement of alkali metals in a 20kW bubbling fluidized bed[J].Fuel,2004,83(7-8):791-797.
[33] GOTTWALD U,MONKHOUSE P,WULGARIS N,et al.In-situ study of the effect of operating conditions and additives on alkali emissions in fluidised bed combustion[J].Fuel Processing Technology,2002,75(3):215-226.
[34] EYK P J V,ASHMAN P J,ALWAHABI Z T,et al.Quantitative measurement of atomic sodium in the plume of a single burning coal particle[J].Combustion & Flame,2008,155(3):529-537.
[35] BLASING Marc,MULLER M.Release of alkali metal,sulphur,and chlorine species from high temperature gasification of high-and low-rank coals[J].Fuel Processing Technology,2013,106(2):289-294.
[36] WALL T F,CREELMAN R A,GUPTA R P,et al.Coal ash fusion temperatures-new characterization techniques,and implications for slagging and fouling[J].Progress in energy and combustion science,1998,24(4):345-353.
[37] KAHRAMAN H,REIFENSTEIN A P,COIN C D A.Correlation of ash behaviour in power stations using the improved ash fusion test[J].Fuel,1999,78(12):1463-1471.
[38] KIM J H,KIM G B,JEON C H.Prediction of correlation between ash fusion temperature of ASTM and thermo-mechanical analysis[J].Applied Thermal Engineering,2017,125:1291-1299.
[39] 刘炎泉.循环流化床燃用新疆准东煤结渣沾污机理及防止研究[D].杭州:浙江大学,2019.
LIU Yanquan.Research on prevention and mechanism of slagging and fouling during Zhundong coal combustion in a circulating fluidized bed[D].Hangzhou:Zhejiang University,2019.
[40] 周广钦.准东煤立式旋风炉燃烧及沾污特性试验研究[D].西安:西安热工研究院有限公司,2018.
ZHOU Guangqin.Experimental study on combustion and contamination characteristics of Zhundong Coal in vertical cyclone furnace[D].Xi’an:Xi’an Thermal Power Research Institute Co.,Ltd.,2018.
[41] STAM A F,LIVINGSTON W R,CREMERS M F G,et al.Review of models and tools for slagging and fouling prediction for biomass co-combustion[A].Workshop on high cofiring percentages in new coal fired power plants[C].Hamburg,2009.
[42] ASTM D.Standard test method for fusibility of coal and coke ash[J].Annual Book of Standards,1975:26.
[43] PANG C H,HEWAKANDAMBY B,WU T,et al.An automated ash fusion test for characterisation of the behaviour of ashes from biomass and coal at elevated temperatures[J].Fuel,2013,103:454-466.
[44] JING N,ZHU M,SHEN G,et al.Effect of ash preparation method on the sintering characteristics of ashes from combustion of coal and biomass blends[J].Fuel,2016,186:830-837.
[45] WANG C,JIN X,WANG Y,et al.Release and transformation of sodium during pyrolysis of Zhundong coals[J].Energy & Fuels,2015,29(1):78-85.
[46] KAHRAMAN H,BOS F,REIFENSTEIN A,et al.Application of a new ash fusion test to Theodore coals[J].Fuel,1998,77(9):1005-1011.
[47] 戴爱军.煤灰成分对灰熔融性影响研究[J].洁净煤技术,2007,13(5):23-26.
DAI Aijun.Reserch on influence of ash components in coal ash on ash fusibility[J].Clean Coal Technology,2007,13(5):23-26.
[48] 许洁,刘霞,李德侠,等.煤灰流动温度预测模型的研究[J].燃料化学学报,2012,40(12):1415-1421.
XU Jie,LIU Xia,LI Dexia,et al.Prediction model for flow temperature of coal ash[J].Journal of Fuel Chemistry and Technology,2012,40(12):1415-1421.
[49] 杨燕梅.准东煤碱/碱土金属赋存形态与释放特性的研究[D].北京:清华大学,2017.
YANG Yanmei.Occurrence modes and release characteristics of alkali and alkaline earth metal species in Zhundong Coals[D].Beijing:Tsinghua University,2017.
[50] HURLEY J P,BENSON S A.Ash deposition at low temperatures in boilers burning high-calcium coals 1.Problem definition[J].Energy & Fuels,1995,9(5):775-781.
[51] DAI B Q,LOW F,GIROLAMO A D,et al.Characteristics of ash deposits in a pulverized lignite coal-fired boiler and the mass flow of major ash-forming inorganic elements[J].Energy & Fuels,2013,27(10):6198-6211.
[52] GUPTA S,GUPTA R,BRYANT G,et al.Characterization of ash deposition and heat transfer behavior of coals during combustion in a pilot-scale facility and full-scale utility[J].Energy & Fuels,2009,23(5):2570-2575.
[53] BABAT S,SPORL R,MAIER J,et al.Investigation of deposit formation and its characterization for a pulverized bituminous coal power plant[J].Fuel Processing Technology,2016,141:225-234.
[54] RUSSELL N V,MENDEZ L B,WIGLEY F,et al.Ash deposition of a Spanish anthracite:Effects of included and excluded mineral matter[J].Fuel,2002,81(5):657-663.
[55] PEREZ M G,FRY A R,VAKKILAINEN E,et al.Ash deposit analysis of the convective section of a pilot scale combustor firing two different sub-bituminous coals[J].Energy & Fuels,2016,30(10):8753-8764.
[56] LI G,LI S,HUANG Q,et al.Fine particulate formation and ash deposition during pulverized coal combustion of high-sodium lignite in a down-fired furnace[J].Fuel,2015,143:430-437.
[57] VORRES.Melting behavior of coal ash materials from coal ash composition[J].Quaternary International,1977,371(2):197-208.
[58] VASSILEV SV,KITANO K,TAKEDA S,et al.Influence of mineral and chemical composition of coal ashes on their fusibility[J].Fuel Processing Technology,1995,45(1):27-51.
[59] LI J,ZHU M,ZHANG Z,et al.The mineralogy,morphology and sintering characteristics of ash deposits on a probe at different temperatures during combustion of blends of Zhundong lignite and a bituminous coal in a drop tube furnace[J].Fuel Processing Technology,2016,149:176-186.
[60] 唐诗,傅培舫,刘洋,等.准东煤及配煤的矿物相变及熔融机理[J].燃烧科学与技术,2019,25(4):324-330.
TANG Shi,FU Peifang,LIU Yang,et al.Mineral phase transformation and ash melting mechanism of Zhundong Coal and blended coal[J].Journal of Combustion Science and Technology,2019,25(4):324-330.
[61] WEE H L,WU H,ZHANG D,et al.The effect of combustion conditions on mineral matter transformation and ash deposition in a utility boiler fired with a sub-bituminous coal[J].Proceedings of the Combustion Institute,2005,30(2):2981-2989.
[62] WU X,ZHANG X,YAN K,et al.Ash deposition and slagging behavior of Chinese Xinjiang high-alkali coal in 3 MWth pilot-scale combustion test[J].Fuel,2016a,181:1191-1202.
[63] CAI Y,TAY K,ZHENG Z,et al.Modeling of ash formation and deposition processes in coal and biomass fired boilers:A comprehensive review[J].Applied energy,2018,230:1447-1544.
[64] WANG H.Modeling of ash formation and deposition in PC fired utility boilers[D].Provo:Brigham Young University,1998.
[65] DESJARDIN P E,PRESSER C,DISIMILE P J,et al.A droplet impact model for agent transport in engine nacelles[A].Proceedings of the Halon Options Technical Working Conference (HOTWC)[C].2002.
[66] ZBOGAR A,FRANDSEN F,JENSEN P A,et al.Shedding of ash deposits[J].Progress in Energy and Combustion Science,2009,35(1):31-56.
[67] KLEINHANS U,WIELAND C,FRANDSEN F J,et al.Ash formation and deposition in coal and biomass fired combustion systems:Progress and challenges in the field of ash particle sticking and rebound behavior[J].Progress in Energy and Combustion Science,2018,68:65-168.
[68] 兰泽全.煤和黑液水煤浆沾污结渣机理及灰沉积动态特性研究[D].杭州:浙江大学,2004.
LAN Zequan.Mechanism & dynamic characteristics of fouling and slagging for coal and black liquor coal-water slurry[D].Hangzhou:Zhejiang University,2004.
[69] VUTHALURU H B.Remediation of ash problems in pulverised coal-fired boilers[J].Fuel,1999,78(15):1789-1803.
[70] LUAN C,YOU C,ZHANG D.An experimental investigation into the characteristics and deposition mechanism of high-viscosity coal ash[J].Fuel,2014,119:14-20.
[71] ZHAN Z,BOOL L E,FRY A,et al.Novel temperature-controlled ash deposition probe system and its application to oxy-coal combustion with 50% inlet O2[J].Energy & Fuels,2014,28(1):146-154.
[72] SONG G,YANG S,SONG W,et al.Release and transformation behaviors of sodium during combustion of high alkali residual carbon[J].Applied Thermal Engineering,2017,122:285-296.
[73] 史航,吴玉新,郭前鑫,等.准东煤碱金属在350 MW煤粉炉内的沿程分布特性[J].中国电机工程学报,2018,38(23):6981-6986,7131.
SHI Hang,WU Yuxin,GUO Qianxin,et al.Distribution characteristics of the alkali in Zhundong Coal along the flue gas of a 350 MW pulverized coal furnace[J].Proceedings of the CSEE,2018,38(23):6981-6986,7131.
[74] JENSEN J R,NIELSEN L B,SCHULTZ-MOLLER C,et al.The nucleation of aerosols in flue gases with a high content of alkali-A laboratory study[J].Aerosol Science & Technology,2000,33(6):490-509.
[75] STEINBERG M,SCHOFIELD K.The controlling chemistry in flame generated surface deposition of Na2SO4 and the effects of chlorine[A].Symposium (International) on Combustion[C].1996,26(2):1835-1843.
[76] KYI S,CHADWICK B L.Screening of potential mineral additives for use as fouling preventatives in Victorian brown coal combustion[J].Fuel,1999,78(7):845-855.
[77] LI J,Zhu M,ZHANG Z,et al.Effect of coal blending and ashing temperature on ash sintering and fusion characteristics during combustion of Zhundong lignite[J].Fuel,2017,195:131-142.
[78] HAUGEN N E L,KRAGSET S.Particle impaction on a cylinder in a crossflow as function of Stokes and Reynolds numbers[J].Journal of Fluid Mechanics,2010,661:239-261.
[79] 李鹏,曾琦,张大德,等.低温燃烧法在燃用准东煤锅炉上的应用[J].华电技术,2014,36(8):30-33,77.
LI Peng,ZENG Qi,ZHANG Dade,et al.Application of low-temperature combustion method in east Junggar coal-fired boiler[J].Huadian Technology,2014,36(8):30-33,77.
