我国的煤炭资源和煤层气资源储量丰富[1-3],随着社会能源需求的增长和资源勘探开发技术的提高,煤层气作为一种清洁的非常规能源,对于缓解我国的能源紧张和促进经济发展有非常重要的作用。我国经过多年的发展已建立了沁水盆地和鄂尔多斯盆地的煤层气产业基地[4],仅沁水盆地的煤层气可采储量就高达325×108 m3[5]。煤层气在煤储层中的存储方式多样,吸附是煤层气最为重要的存储方式之一[6-7],因此,正确认识储层条件下煤层气的吸附规律对于准确评价煤层气储量及合理制定煤层气的开发方案具有非常重要的意义。
煤层的沉积及煤层气的生成和吸附均是在水生环境下进行的[8],经过注水和压裂改造后,外来流体在煤层中也有不同程度的滞留[9],因此,基于干煤样等温吸附实验得出的数据并不能用于准确评价实际储层中煤层气的含量。随着对甲烷在煤岩中吸附的逐步研究和认识,水分会影响煤岩的吸附性能这一观点被普遍接受。COPPENS通过采用比利时的煤岩进行实验发现当煤样的含水率为2.85%时,甲烷的吸附量下降了3%[10]。JOUBERT等通过含水煤样吸附实验研究发现煤岩中的水分使得甲烷吸附量明显降低,而当含水率增加至某一特定值时,其对甲烷的吸附量不再产生影响[11]。桑树勋等通过注水煤样吸附实验发现,液态水可提高甲烷在煤岩中的吸附量[12]。张时音等通过分析发现注水煤样中液态水比平衡水煤样中的水分对甲烷的吸附影响更显著[13]。黄丹等的研究表明水分对高阶煤的吸附影响较小,而注水煤样中的液态水增加了甲烷的吸附量[14]。赵东等通过块状煤岩吸附动力学实验研究发现干燥煤样的吸附速率是含水煤样吸附速率的16~22倍[15]。焦斌通过分子间作用力的计算表明水分子与煤体表面的极性官能团相互作用形成作用力较强的氢键,使得水分子优先吸附于煤岩表面从而降低了甲烷的吸附量[16]。魏迎春等开展了不同矿化度水对煤岩吸附性能影响的实验研究,结果表明矿化度水比蒸馏水对煤岩吸附的影响更大[17]。
有关研究还发现煤层气的吸附会使得煤岩基质发生膨胀和收缩。HARPALANI发现煤层气吸附会引起煤基质发生膨胀,而解吸引起煤基质收缩从而使得煤岩渗透率发生变化[18]。CZAPLINSKI和GUSTKIEWICZ等发现CO2在煤岩中的吸附也会引起煤岩发生形变[19-21]。ZHOU等通过扫描电镜实验研究发现在吸附时煤基质膨胀形变介于5%~7%,解吸时残余形变介于0.5%~3.0%[22]。此外,深部煤层的温度高于甲烷的临界温度,即实际储层中甲烷气的吸附往往发生在超临界条件下[23-25]。在超临界条件下体系中不会发生气液转化,在煤层中吸附态的甲烷呈类液态[26],不存在饱和蒸汽压[27-28]。
目前对于甲烷在含水煤样中的吸附模型的研究较少,现有的研究中采用最多的依然为经典的Langmuir模型。李树刚等认为甲烷在含水煤样中的吸附满足Langmuir模型,且Langmuir吸附常数a与含水率呈三次多项式关系,吸附常数b与含水率呈线性关系[29]。林海飞等采用Langmuir模型对含水煤岩的吸附数据进行拟合分析,发现吸附常数a与含水率为线性关系,吸附常数b与含水率无明显的相关性[30]。田伟兵等通过开展含水煤岩的吸附解吸实验并用Langmuir模型对吸附数据进行了拟合,拟合精度较高[31]。CROSDALE等的研究表明Langmuir模型不符合煤岩超微孔中的吸附机理,也不能描述等温吸附曲线上高压段出现的最大值的现象[32]。SAKUROVS用气体密度代替Langmuir模型和Dubinin-Radushkevich(D-R)模型中的压力项,使得吸附模型适用于煤层气的超临界吸附[28]。
由此可见,含水条件下煤层气的吸附过程是一个受多种因素影响的复杂过程,而目前含水煤岩等温吸附模型选用较为单一。为了准确描述甲烷在含水煤岩中的吸附特征,应选用考虑煤岩膨胀变形、超临界吸附条件、煤层气溶解[27,33-34]等因素在内的更准确的吸附模型。因此,笔者通过开展不同温度下不同含水率煤岩的等温吸附实验,并采用适用于超临界条件的Langmuir模型、Dubinin-Radushkevich(D-R)模型、Dubinin-Astakhov(D-A)模型,以及引入了综合修正因子k的Langmuir+k模型、Dubinin-Radushkevich+k(D-R+k)模型、Dubinin-Astakhov+k(D-A+k)模型分别对不同温度下含水煤岩的吸附数据进行了拟合及对比分析,并就水分和温度对甲烷在煤岩中吸附的共同影响做了进一步的分析讨论。
1 等温吸附实验
1.1 实验设备及条件
实验设备:本实验所用设备为中国石油大学(华东)油气渗流中心非常规储层岩石物性评价实验室中的高精度高温高压煤岩等温吸附测量仪YRD-HPHTsor,该设备基于容量法,最高耐压28 MPa,精度0.001 MPa,最高耐温可达90 ℃,精度0.01 ℃。设备主要由气源、气体增压泵、高压缓冲容器、参考室、样品室、恒温油浴、真空泵及数据采集系统组成,设备流程如图1所示。

图1 实验设备流程
Fig.1 Flow chart of experiment equipment
实验样品。样品为来自于山西某矿的无烟煤,其基础物性见表1,由物性测量结果可知该样品中固定碳占79.33%,挥发分和灰分各占7.05%和12.31%,镜质组占78.21%,惰质组占16.68%。按照行业标准GB/T 474将上述煤样制备成60~80目的粉末样品,并制作不同含水率的湿样。实验所用气体为高纯氦气和高纯甲烷(纯度为99.99%)。
表1 煤样的组成及工业分析和元素分析结果
Table 1 Results of composition, proximate analysis and elemental analysis of the coal sample %
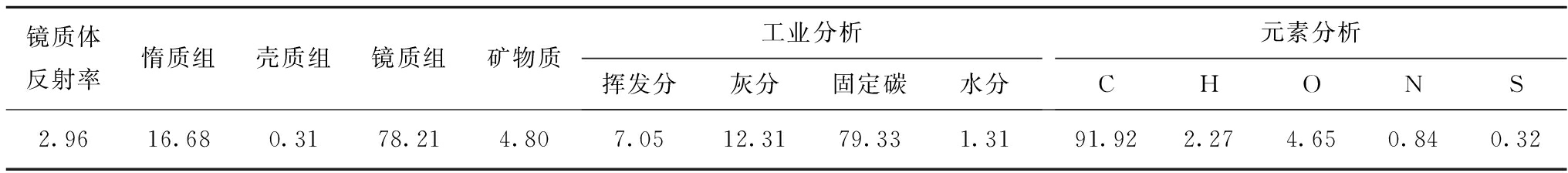
镜质体反射率惰质组壳质组镜质组矿物质工业分析挥发分灰分固定碳水分元素分析CHONS2.9616.680.3178.214.807.0512.3179.331.3191.922.274.650.840.32
实验条件。实验温度通过油浴进行调控,分别在30和60 ℃条件下开展等温吸附实验,实验压力为0~15 MPa,压力平衡时间为12 h。
1.2 实验方法
(1)样品制备。将块状煤岩样品通过粉碎、研磨和筛选制得60~80目的粉末样品,在105 ℃下干燥6 h制得干样。然后向干燥煤岩样品雾化喷洒不同含量的水分并充分搅拌,使得样品含水均匀,之后将湿样置于恒温恒湿箱中进行湿样处理,每隔24 h称量1次,直到相近2次称量的质量变化低于2%,则可制得不同含水率的湿样[31]。含水率计算公式为
![]()
(1)
式中,Wt为样品的含水率,%;md为样品的干质量,g;mw为样品的湿质量,g。
(2)等温吸附实验。利用YRD-HPHTsor等温吸附仪,依据行业标准GB/T 19560—2008开展煤岩的等温吸附实验。最高实验压力为15 MPa,实验压力测试点不少于8个,甲烷吸附量计算过程中气体的压缩因子根据Setzmann-Wagner状态方程[35]计算求得。
2 超临界条件下煤岩等温吸附模型
常用于描述甲烷在煤岩和页岩中吸附的模型主要有基于单层吸附的Langmuir模型及基于微孔填充的D-R 模型和D-A模型。实验测量所得的吸附量为过剩吸附量而非绝对吸附量,即该吸附量的计算并未考虑吸附态甲烷体积的影响,因此需将上述的经典模型进行修正以适用于拟合实际测量数据。且考虑到煤层气的吸附为超临界吸附,按照SAKUROVS[28]和THOMAS等[36]的研究,使用密度代替各吸附模型中的压力项,则修正后的Langmuir模型、D-R模型以及D-A模型如式(2)~(4)所示:
![]()
(2)
![]()
(3)
![]()
(4)
式中,Nex为过剩吸附量,mmol/g;Nm为饱和吸附量,mmol/g;ρg为自由相甲烷密度,kg/m3;ρL为吸附量等于1/2饱和吸附量时对应的甲烷气体密度,kg/m3;ρa为吸附相甲烷的密度,多数研究中此参数的取值为液态甲烷密度[33],本研究中亦对其取值为液态甲烷密度,即421 kg/m3;D为气体和吸附剂之间亲和系数;n为吸附剂结构的非均质系数。
由前述可知甲烷在含水煤岩中的吸附受多种因素的影响,为了能够更准确的描述实际储层条件下的煤层气吸附过程,SAKUROVS等[28,37]在吸附模型中引入综合修正因子k对吸附过程中煤层气的溶解、煤岩的膨胀收缩变形、自由空间体积测量误差等进行修正,以提高模型的拟合精度。基于此修正方法对式(2)~(4)进行进一步修正,得到Langmuir+k模型、D-R+k模型及D-A+k模型,如式(5)~(7)所示:
![]()
(5)
![]()
(7)
3 实验结果及分析
3.1 不同超临界吸附模型拟合结果分析
分别采用式(2)~(7)所示的Langmuir模型、D-R模型、D-A模型、Langmuir+k模型、D-R+k模型、D-A+k模型分别对30和60 ℃条件下不同含水率煤岩样品吸附实验结果进行拟合,通过均方根误差ERMS来评价其拟合质量,ERMS越低,表明拟合精度越高。拟合参数见表2和3,不同模型的拟合结果如图2所示。
表2 不同吸附模型对30 ℃下湿煤样中甲烷吸附的拟合参数
Table 2 Fitting parameters of different adsorption models for methane adsorption on wet coal at 30 ℃

模型含水率Wt/%Nm/(mmol·g-1)ρL/(kg·m-3)DnkERMS01.8147.289———0.061 750Langmuir2.971.93613.380———0.058 3306.211.91916.080———0.051 96001.4534.555——0.003 6710.008 743Langmuir+k2.971.4057.178——0.004 3930.009 5906.211.3578.855——0.004 3760.009 26601.933—0.047 910——0.033 290D-R2.971.972—0.060 990——0.032 9206.211.929—0.068 130——0.025 75001.918—0.047 530—0.001 4070.035 020D-R+k2.971.784—0.054 780—0.001 5880.028 3506.211.746—0.062 300—0.001 5160.020 28001.887—0.037 0102.156—0.033 330D-A2.972.043—0.080 9101.816—0.032 5706.212.003—0.089 4101.825—0.024 32001.415—0.004 6473.2850.003 6310.007 889D-A+k2.971.323—0.007 0203.2050.004 4940.007 2156.211.303—0.013 2302.8830.004 2960.009 100
表3 不同吸附模型对60 ℃下湿煤样中甲烷吸附的拟合参数
Table 3 Fitting parameters of different adsorption models for methane adsorption on wet coal at 60 ℃

模型含水率Wt/%Nm/(mmol·g-1)ρL/(kg·m-3)DnkERMS01.71911.150———0.054 240Langmuir2.421.79116.840———0.048 1205.841.88125.210———0.034 92001.2466.136——0.004 5130.007 421Langmuir+k2.421.1988.541——0.004 9410.009 5525.841.18613.130——0.005 0420.009 51601.796—0.058 93——0.018 850D-R2.421.804—0.070 36——0.021 0905.841.808—0.084 91——0.019 01001.679—0.055 52—0.001 1030.015 410D-R+k2.421.591—0.063 54—0.001 8960.011 9705.841.538—0.075 99—0.002 2770.007 32101.839—0.070 841.888—0.018 350D-A2.421.918—0.103 701.759—0.016 0105.841.971—0.133 901.711—0.008 96401.287—0.013 302.7740.003 7870.002 708D-A+k2.421.262—0.023 222.5480.004 0180.007 4735.841.255—0.039 672.3500.004 0540.007 273

图2 含水煤岩超临界吸附模型拟合对比
Fig.2 Fitting results comparison of different supercritical adsorption models for wet coal
由图2可知,6种不同模型拟合效果各不相同,在初始的低压阶段(自由甲烷密度小于20 kg/m3),不同模型均有较好的拟合效果,拟合值与实测值接近。而当压力继续增加时,各模型的拟合效果出现比较明显的差别,在中压阶段(甲烷密度介于20~80 kg/m3)和高压阶段(甲烷密度高于80 kg/m3),不同吸附模型的拟合结果各不相同。为了能够更清楚地观察各吸附模型拟合效果的差别,将各等温吸附曲线的中、高压阶段进行局部放大,可见在高压阶段实测的吸附量呈现轻微的下降趋势,以30 ℃下干燥煤样的吸附量下降最为明显,其他学者的研究中也发现了这一现象[38-39]。由局部放大后的曲线可见考虑吸附相体积修正后的6种模型均能反映出高压阶段吸附量下降的趋势,但Langmuir模型、D-R模型和D-A模型在中压阶段偏高,而在高压阶段的下降幅度更大,拟合值偏低,与实测吸附曲线的趋势有非常明显的差别。而引入综合修正因子k的Langmuir+k,D-R+k及D-A+k模型拟合效果更好,其中Langmuir+k模型和D-A+k模型拟合结果与实测吸附结果最为接近。HE等[38]采用了修正煤岩膨胀的D-R模型拟合亚临界和超临界条件下CO2在干煤样中的吸附时也取得了较好的拟合效果。SAKUROVS等[28]应用不同吸附模型对超临界条件下不同气体在干燥煤样中的吸附进行拟合,表明考虑煤基质膨胀的D-R模型拟合效果更好。由此可见,考虑煤岩中甲烷气体的溶解、由吸附和水分引起的煤岩膨胀等因素在内的修正模型更适合表征含水煤岩超临界吸附。
为了定量评价上述模型对含水煤岩超临界吸附数据的拟合效果,本研究中以均方根误差ERMS作为评价标准。由表2和3的拟合结果可知,6种模型分别对30 ℃和60 ℃下不同含水率的煤岩吸附进行拟合,其ERMS有较大的差别,如图3所示。Langmuir,D-R,D-A,D-R+k模型的ERMS介于0.01~0.06,而Langmuir+k和D-A+k模型的ERMS却减小一个量级,其值介于0.002~0.009,且D-A+k模型的ERMS更小。由此可见,基于微孔填充理论的D-A+k模型对于含水煤岩超临界甲烷吸附的拟合精度更高,拟合质量更好,这与图2所示的拟合曲线效果相一致。有关干燥煤样的吸附研究也指出基于微孔充填理论的D-A 模型更适合描述甲烷和二氧化碳在煤层中的吸附[40-41]。

图3 不同吸附模型拟合结果的ERMS值
Fig.3 ERMS of fitting results by different models
3.2 水分和温度对煤岩吸附的综合影响
由上述探讨可知,相比其他几种模型,D-A+k吸附模型对含水煤岩超临界吸附特征的表征最准确,以下根据D-A+k吸附模型的拟合结果来讨论水分和温度对煤岩吸附能力的综合影响。30和60 ℃条件下不同含水率煤岩的饱和吸附量与含水率的关系如图4所示。
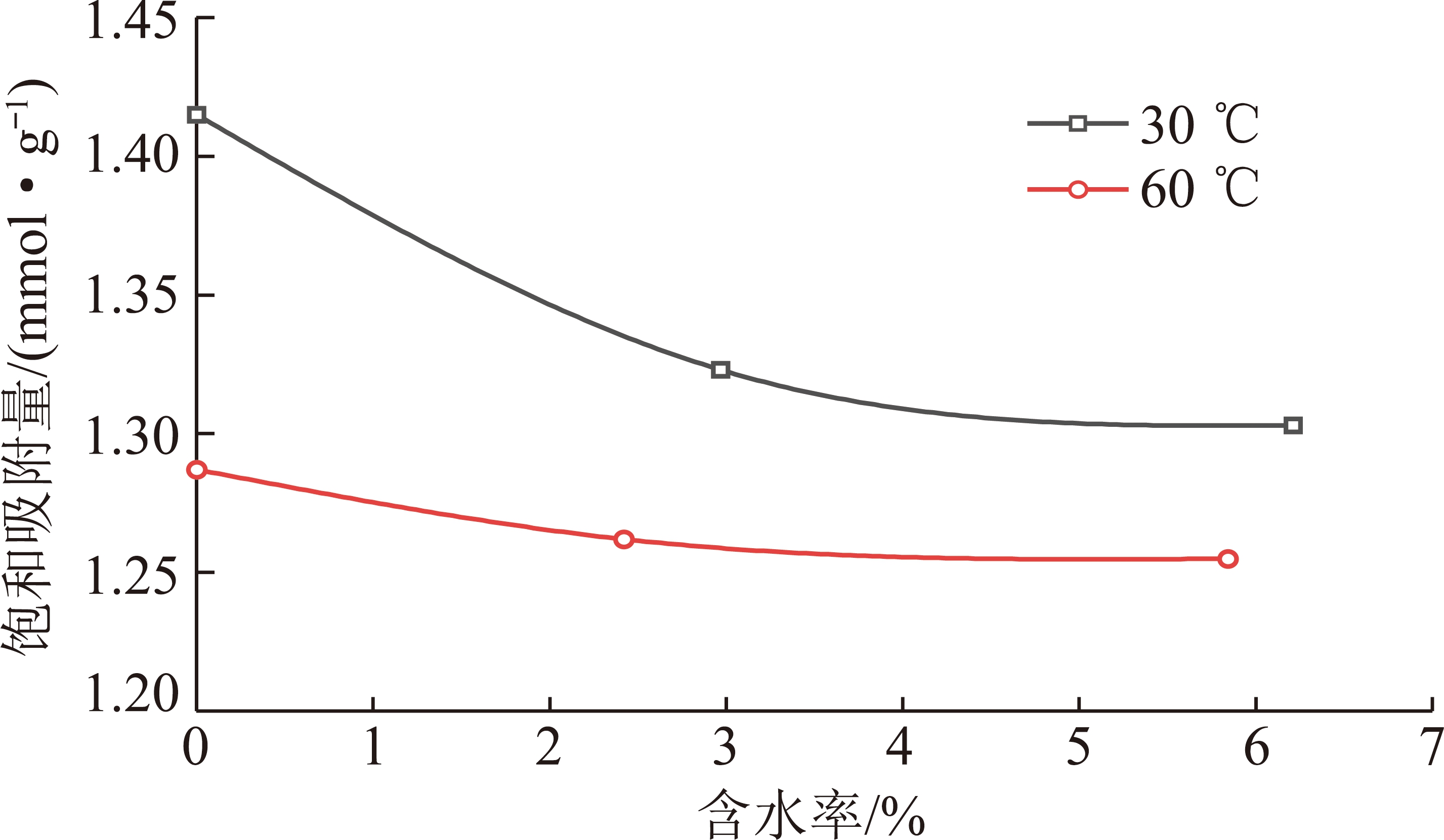
图4 饱和吸附量与含水率的关系
Fig.4 Relationship between saturated adsorption capacity
and water content
不同温度条件下煤岩的饱和吸附量均随着含水率的增加而下降,这是由于吸附过程中水分子与甲烷分子在煤岩表面存在竞争吸附,由于水分子是极性分子,易与煤岩表面的官能团结合形成氢键[16],使得水分子与煤岩表面的作用力强于甲烷分子与煤岩表面的作用力,因此水分子会优先吸附于煤岩表面而占据部分甲烷分子的吸附位,使得甲烷的吸附量明显降低。其次,水分子进入煤岩孔隙后会在煤岩表面形成水膜,减少甲烷与煤岩表面的接触面积,进一步降低了甲烷的吸附量。此外,水会在煤岩的孔隙中产生毛细现象使得煤岩中的部分孔隙被水填充[42],当孔隙的内部与外部环境之间的压差小于毛细阻力时,甲烷分子无法进入孔隙内部,而甲烷分子的吸附位集中分布在煤岩孔隙的内表面,因此孔隙中水的出现降低了甲烷的吸附量。
含水率由0增加至2%~3%时,甲烷在煤岩中的吸附量明显下降,而当含水率继续增加时,饱和吸附量变化较小。30 ℃条件下煤岩的含水率由0增加至2.97%时,饱和吸附量由1.415 mmol/g降至1.323 mmol/g,降幅为6.5%,60 ℃条件下煤岩含水率由0增加至2.42%时,其饱和吸附量由1.287 mmol/g降至1.262 mmol/g,降幅为2%。当煤岩的含水率高于2%~3%时,水分对甲烷吸附量的影响减小,这意味着存在一个临界含水率值,当含水率高于该临界值时甲烷吸附量受水分影响减小。由此可见,水对煤岩吸附性能的影响呈非线性衰减[43]。郭淑敏等也指出当煤岩的含水率高于2.35%后,甲烷的饱和吸附量对含水率不再敏感[44]。
温度对煤层气的吸附也有非常重要的影响,由图4可知,不同含水率的煤岩在60 ℃下的甲烷吸附量始终低于30 ℃下的甲烷吸附量。这是由于甲烷在煤岩中的吸附为物理吸附,属于放热过程,温度较高时甲烷分子的热运动更加剧烈,使得吸附于煤岩表面的甲烷分子更容易脱附,因此随着温度的升高甲烷的吸附量降低。
图4所示的结果表明煤岩对甲烷的吸附同时受含水率和温度的综合影响。当温度由30 ℃升高至60 ℃时,干煤样的饱和吸附量由1.415 mmol/g降至1.287 mmol/g,降幅为9.05%,而含水率约为2.5%的湿煤样,饱和吸附量由1.323 mmol/g降至1.262 mmol/g,降幅为4.61%,含水率约为6.0%的煤样饱和吸附量由1.303 mmol/g降至1.255 mmol/g,降幅为3.68%。由此可见,含水率越高的煤样其饱和吸附量受温度的影响越小,在低阶煤的吸附研究中也有相似的现象[39]。此外,随着温度的升高,干煤样和含水煤样的饱和吸附量之差减小,即温度越高,煤岩的吸附能力受水分的影响也越小,30 ℃下含水率由0增至6.21%时饱和吸附量下降了7.92%,而60 ℃下含水率由0增加至5.84%时,饱和吸附量下降了2.49%。这可能是由于升高温度时,煤岩中部分被水分子占据的吸附位被释放出来使得水分对甲烷的吸附影响变小[45]。
4 结 论
(1)使用经典吸附模型拟合含水煤岩超临界吸附时,在中、高压阶段误差较大,在经典的吸附模型中引入考虑煤岩中的溶解气、煤岩膨胀变形等因素的综合修正因子k可以有效提高吸附模型的拟合精度。
(2)基于微孔填充理论的D-A+k模型更适合描述含水煤岩超临界吸附的特征,对于实测数据拟合误差更小,拟合精度更高。
(3)含水煤岩的吸附能力受温度和含水率的综合影响,饱和吸附量随温度的升高而降低,随含水率的升高先降低而后趋于稳定,含水率越高的煤岩其饱和吸附量受温度影响越小,温度较高时饱和吸附量受含水率的影响较小。
[1] 朱志敏,杨春,沈冰,等.煤层气及煤层气系统的概念和特征[J].新疆石油地质,2006,27(6):763-765.
ZHU Zhimin,YANG Chun,SHEN Bing,et al.The definition and characteristics of coalbed methane and coalbed methane system[J].Xingjiang Petroleum Geology,2006,27(6):763-765.
[2] 刘见中,孙海涛,雷毅,等.煤矿区煤层气开发利用新技术现状及发展趋势[J].煤炭学报,2020,45(1):258-267.
LIU Jianzhong,SUN Haitao,LEI Yi,et al.Current situation and development trend of coalbed methane development and utilization technology in coal mine area[J].Journal of China Coal Society,2020,45(1):258-267.
[3] 许耀波,郭盛强.软硬煤复合的煤层气水平井分段压裂技术及应用[J].煤炭学报,2019,44(4):1169-1177.
XU Yaobo,GUO Shengqiang.Technology and application of staged fracturing in coalbed methane horizontal well of soft and hard coal composite coal seam[J].Journal of China Coal Society,2019,44(4):1169-1177.
[4] 朱庆忠,杨延辉,左银卿,等.对于高煤阶煤层气资源科学开发的思考[J].天然气工业,2020,40(1):55-60.
ZHU Qingzhong,YANG Yanhui,ZUO Yinqing,et al.On the scientific exploitation of high-rank CBM resources[J].Natural Gas Industry,2020,40(1):55-60.
[5] QING Y,WANG C,DING Q,et al.Impact of surfactant in fracturing fluid on the adsorption-desorption processes of coalbed methane[J].Journal of Natural Gas Science and Engineering,2015,26:35-41.
[6] YORO K O,KAMOSA M,SEKOAI P T,et al.Modelling and experimental investigation of effects of moisture and operating parameters during the adsorption of CO2 onto polyaspartamide[J].International Journal of Coal Science & Technology,2019,6(2):225-234.
[7] CHENG Y,JIANG H,ZHANG X,et al.Effects of coal rank on physicochemical properties of coal and on methane adsorption[J].International Journal of Coal Science & Technology,2017,4(2):129-146.
[8] 朱苏阳,李传亮,杜志敏,等.煤层气的复合解吸模式研究[J].中国矿业大学学报,2016,45(2):316-324.
ZHU Suyang,LI Chuanliang,DU Zhimin,et al.Compound desorption model of coalbed methane[J].Journal of China University of Mining & Technology,2016,45(2):316-324.
[9] 王凤林,袁玉,张遂安,等.不同含水及负压条件下煤层气等温吸附解吸规律[J].煤炭科学技术,2019,47(6):158-163.
WANG Fenglin,YUAN Yu,ZHANG Suian,et al.Isothermal adsorption and desorption of coalbed methane under different water saturation and negative pressure[J].Coal Science and Technology,2019,47(6):158-163.
[10] COPPENS L.L’adsorption du méthane par les houilles sous pression élevée[A].Annales des Mines de Belgique[C].Belgique,1936,37:173.
[11] JOUBERT J I,GREIN C T,BIENSTOCK D.Sorption of methane in moist coal[J].Fuel,1973,52(3):181-185.
[12] 桑树勋,朱炎铭,张 井,等.液态水影响煤吸附甲烷的实验研究:以沁水盆地南部煤储层为例[J].科学通报,2005,50(S1):70-75.
SANG Shuxun,ZHU Yanming,ZHANG Jing,et al.Influence of liquid water on coalbed methane adsorption:An experimental research on coal reservoirs in the south of Qinshui Basin[J].Chinese Science Bulletin,2005,50(S1):70-75.
[13] 张时音,桑树勋.液态水影响不同煤级煤吸附甲烷的差异及其机理[J].地质学报,2008,82(10):1350-1354.
ZHANG Shiyin,SANG Shuxun.Influence mechanism of liquid water on methane absorption of coals with different ranks[J].ACTA Geologica Sinica,2008,82(10):1350-1354.
[14] 黄丹,夏大平,徐涛,等.水分和粒度对煤吸附甲烷性能的实验研究[J].中国煤炭地质,2013,25(7):22-25.
HUANG Dan,XIA Daping,XU Tao,et al.An Experimental research on methane adsorption performance impacted from moisture and granularity[J].Coal Geology of China,2013,25(7):22-25.
[15] 赵东,冯增朝,赵阳升.基于吸附动力学理论分析水分对煤体吸附特性的影响[J].煤炭学报,2014,39(3):518-523.
ZHAO Dong,FENG Zengchao,ZHAO Yangsheng.Effects of liquid water on coalbed methane adsorption characteristics based on the adsorption kinetic theory[J].Journal of China Coal Society,2014,39(3):518-523.
[16] 焦彬.平衡水条件下煤体瓦斯吸附特性的研究及应用[D].徐州:中国矿业大学,2016:60-61.
JIAO Bin.Research and application on methane adsorption characteristic under equilibrium water condition[D].Xuzhou:China University of Mining & Technology,2016:60-61.
[17] 魏迎春,项歆璇,王安民,等.不同矿化度水对煤储层吸附性能的影响[J].煤炭学报,2019,44(9):2833-2839.
WEI Yingchun,XIANG Xinxuan,WANG Anmin,et al.Influence of water with different salinity on the adsorption performance of coal reservoir[J].Journal of China Coal Society,2019,44(9):2833-2839.
[18] HARPALANI S,SCHRAUFNAGEL R A.Shrinkage of coal matrix with release of gas and its impact on permeability of coal[J].Fuel,1990,69(5):551-556.
[19] CZAPLINSKI A,GUSTKIEWICZ J.Sorption stresses and deformations in coal[J].Strata Multiphase Medium,1990,2:455-468.
[20] GUSTKIEWICZ J,ORENGO Y.Behavior of coal caused by water or carbon dioxide[M].Hackensack:World Scientific Publishing,1998:18-27.
[21] MOHANY M M,PAL B K.Sorption behavior of coal for implication in coal bed methane an overview[J].International Journal of Mining Science and Technology,2017,27(2):307-314.
[22] ZHOU D,FENG Z,ZHAO D,et al.Experimental study of meso-structural deformation of coal during methane adsorption-desorption cycles[J].Journal of Natural Gas Science and Engineering,2017,42:243-251.
[23] 杨兆彪,秦勇,高弟,等.超临界条件下煤层甲烷视吸附量、真实吸附量的差异及其地质意义[J].天然气工业,2011,31(4):13-16,122.
YANG Zhaobiao,QIN Yong,GAO Di,et al.Difference between apparent and true adsorption quantity of coalbed methane under supercritical conditions and their geology significance[J].Natural Gas Industry,2011,31(4):13-16,122.
[24] 侯晓伟,王猛,刘宇,等.页岩气超临界状态吸附模型及其地质意义[J].中国矿业大学学报,2016,45(1):111-118.
HOU Xiaowei,WANG Meng,LIU Yu,et al.Supercritical adsorption model of shale gas and its geological significance[J].Journal of China University of Mining & Technology,2016,45(1):111-118.
[25] 周理,李明,周亚平.超临界甲烷在高表面活性炭上的吸附测量及其理论分析[J].中国科学(B辑),2000,30(1):49-56.
ZHOU Li,LI Ming,ZHOU Yaping.Adsorption measurement and theoretical analysis of supercritical methane on high surface area active carbon[J].Science in China (Series B),2000,30(1):49-56.
[26] PALMER I,VAZIRI H.Permeability changes in a CBM reservoir during production:An update and implications for CO2 injection[A].Proceedings of the 2004 International Coalbed Methane Symposium[C].Tuscaloosa,Alabama,2004:403.
[27] 盛茂,李根生,陈立强,等.页岩气超临界吸附机理分析及等温吸附模型的建立[J].煤炭学报,2014,39(S1):179-183.
SHENG Mao,LI Gensheng,CHEN Liqiang,et al.Mechanism analysis of shale-gas supercritical adsorption and modeling of isorption adsorption[J].Journal of China Coal Society,2014,39(S1):179-183.
[28] SAKUROVS R,DAY S,WEIR S,et al.Application of a modified Dubinin-Radushkevich equation to adsorption of gases by coals under supercritical conditions[J].Energy & Fuels,2007,21(2):992-997.
[29] 李树刚,赵鹏翔,潘宏宇,等.不同含水量对煤吸附甲烷的影响[J].西安科技大学学报,2011,31(4):379-382,387.
LI Shugang,ZHAO Pengxiang,PAN Hongyu,et al.Effect of moisture on adsorption of methane on coal[J].Journal of Xi’an University of Science and Technology,2011,31(4):379,382,387.
[30] 林海飞,姚飞,李树刚,等.温度及含水量对煤吸附甲烷特性影响的实验研究[J].煤矿开采,2014,19(3):9-12.
LIN Haifei,YAO Fei,LI Shugang,et al.Experiment of coal’s temperature and water content influencing methane absorption quality[J].Coal Mining Technology,2014,19(3):9-12.
[31] 田伟兵,李爱芬,韩文成.水分对煤层气吸附解吸的影响[J].煤炭学报,2017,42(12):3196-3202.
TIAN Weibing,LI Aifen,HAN Wencheng.Effect of water content on adsorption/desorption of coalbed methane[J].Journal of China Coal Society,2017,42(12):3196-3202.
[32] CROSDALE P J,BEAMISH B,VALIX M.Coalbed methane sorption related to coalcomposition[J].International Journal of Coal Geology,1998,35(1):147-158.
[33] ZHANG R,LIU S.Experimental and theoretical characterization of methane and CO2 sorption hysteresis in coals based on Langmuir desorption[J].International Journal of Coal Geology,2017,171:49-60.
[34] WEISHAUPTOV Z,MEDEK J,KOV
Z,MEDEK J,KOV
![]() L.Bond forms of methane in porous system of coal II[J].Fuel,2004,83(13):1759-1764.
L.Bond forms of methane in porous system of coal II[J].Fuel,2004,83(13):1759-1764.
[35] SETZMANN U,WAGNER W.A new equation of state and tables of thermodynamic properties for methane covering the range from the melting line to 625 K at pressures up to 1 000 MPa[J].Journal of Physical and Chemistry Reference Data,1991,20(6):1061-1155.
[36] THOMAS F T R,BENHAM M J,APLIN A C,et al.Methane adsorption on shale under simulated geological temperature and pressure condition[J].Energy & Fuels,2013,27(6):3099-3109.
[37] SAKUROVS R,DAY S,WEIR S,et al.Temperature dependence of sorption of gases by coals and charcoals[J].International Journal of Coal Geology,2007,73(3-4):250-258.
[38] HE J,SHI Y,AHN S,et al.Adsorption and desorption of CO2 on Korean coal under subcritical to supercritical conditions[J].The Journal of Physical Chemistry B,2010,114(14):4854-4861.
[39] XIONG J,LIU X J,LIANG L X,et al.Investigation of methane adsorption on chlorite by grand canonical Monte Carlo simulations[J].Petroleum Science,2017,14(1):37-49.
[40] 徐铭,邹德龙.煤顶底板破碎岩体对CO2气体吸附特性的实验研究[J].煤矿安全,2013,44(11):15-17,21.
XU Ming,ZOU Delong.Experimental research of coal roof and floor broken rock on CO2 adsorption characteristics[J].Safety in Coal Mines,2013,44(11):15-17,21.
[41] 周来,冯启言,陈中伟,等.煤层对CO2处置能力评价的修正方法与应用[J].地球与环境,2008,36(4):363-367.
ZHOU Lai,FENG Qiyan,CHEN Zhongwei,et al.A corrected method for the evaluation of carbon dioxide sequestration in the coal seams and its application[J].Earth and Environment,2008,36(4):363-367.
[42] 杨萌萌,袁梅,徐林,等.煤的瓦斯放散初速度影响因素实验现状研究[J].煤炭技术,2016,35(2):168-170.
YANG Mengmeng,YUAN Mei,XU Lin,et al.Study of experimental situation of influence factors on initial speed of methane diffusion[J].Coal Technology,2016,35(2):168-170.
[43] CROSDALE P J,MOORE T A,MARES T E.Influence of moisture content and temperature on methane adsorption isotherm analysis for coals from a low-rank,biogenically-sourced gas reservoir[J].International Journal of Coal Geology,2008,76(1-2):166-174.
[44] 郭淑敏,段小群,徐成法.煤储层条件下平衡湿度测定方法研究[J].焦作工学院学报(自然科学版),2004,23(2):157-160.
GUO Shumin,DUAN Xiaoqun,XU Chengfa.Moisture content under coal reservoir condition-study on the determination method of balance moisture[J].Journal of Jiaozuo Institute of Technology (Natural Science),2004,23(2):157-160.
[45] BUSCH A,GENSTERBLUM Y.CBM and CO2-ECBM related sorption processes in coal:A review[J].International Journal of Coal Geology,2011,87(2):49-71.
