瓦斯,又叫煤层气,是一种优质的清洁能源,同时也是温室气体。我国的煤层气储量十分丰富,进行煤层气的开发不仅可以获得优质的清洁能源,亦可以达到降低矿井瓦斯灾害、节能减排的三重效果[1]。但我国煤层地质条件比较复杂,煤层渗透性低、煤层气易吸附难解吸,严重威胁矿井的安全生产[2]。我国现有低渗煤层增透技术主要有:水力压裂、深孔预裂爆破、高能气体压裂等物理增透技术[3-5],其中以水力压裂技术最为常用[3]。
除了上述物理增透技术,煤矿安全生产领域的学者们也对化学方法改善煤层渗透性开展了相关研究。如郭红玉等使用二氧化氯对煤体进行改性,结果表明具有氧化性能的二氧化氯可部分溶蚀煤层表面,一定程度上提高煤层渗透性[6]。李胜、倪小明等用不同组分的酸液对不同煤阶的煤样进行处理,结果表明酸液通过溶蚀煤中碳酸盐矿物,也能提高煤样渗透性[7-8]。采用化学手段改善煤层渗透性虽已取得了一定的效果,但增透作用尚不理想,而且所用药剂如二氧化氯、盐酸均具有一定腐蚀性与毒性,在其使用过程不可避免地对环境和人体造成危害,所以寻求高效、绿色的化学药剂对煤储层进行增透对于矿井的安全生产和环境保护更具现实意义。
过硫酸铵因具有较高的稳定性与环境友好性,在石油开采领域常作为压裂液的破胶剂,但常温条件下因过硫酸铵未被有效活化,其氧化破胶能力较差[9]。近年来,基于过硫酸盐活化产生硫酸根自由基![]() 的高级氧化技术及在污染土壤原位修复领域的应用被广泛研究[10-11],其相关成果可为过硫酸铵的高效活化提供实验依据。目前,过硫酸铵的活化方式主要有热活化、紫外光活化、超声活化、过渡金属离子活化等。但以上方法均存在一定局限性,如热活化、紫外光活化需要专门的设备以及大量的能源消耗;均相过渡金属离子活化存在回收与分离困难,易产生二次污染等问题[10]。相反,非均相活化剂不仅易于从处理溶液中分离,且可多次循环使用仍能保持较高的活化性能,较好地解决了均相体系过渡金属离子回收与分离困难、金属离子残余对环境造成潜在危害等问题,被认为是一种绿色、高效的过硫酸铵活化方法[12]。
的高级氧化技术及在污染土壤原位修复领域的应用被广泛研究[10-11],其相关成果可为过硫酸铵的高效活化提供实验依据。目前,过硫酸铵的活化方式主要有热活化、紫外光活化、超声活化、过渡金属离子活化等。但以上方法均存在一定局限性,如热活化、紫外光活化需要专门的设备以及大量的能源消耗;均相过渡金属离子活化存在回收与分离困难,易产生二次污染等问题[10]。相反,非均相活化剂不仅易于从处理溶液中分离,且可多次循环使用仍能保持较高的活化性能,较好地解决了均相体系过渡金属离子回收与分离困难、金属离子残余对环境造成潜在危害等问题,被认为是一种绿色、高效的过硫酸铵活化方法[12]。
笔者采用自制的非均相钴基活化剂(Co-NCP)活化过硫酸铵(APS)水溶液,通过对不同变质程度的煤样进行加压浸泡处理,研究其增透效果,为Co-NCP/APS体系提高低渗煤层渗透性的应用提供实验支撑。
1.1.1 煤样
本文实验煤样分别采自河南焦作九里山矿的无烟煤、安阳主焦矿的焦煤、义马杨村矿的长焰煤。其物理参数见表1。
表1 实验煤样物理参数
Table 1 Physical parameters of experimental coal samples

煤样工业分析/%MadAadVdaf真密度/(g·cm-3)视密度/(g·cm-3)坚固性系数长焰煤3.5715.2847.261.371.280.81焦煤0.7711.8928.251.471.351.15无烟煤1.4311.369.131.621.411.35
1.1.2 试剂与钴基活化剂的制备
试剂:过硫酸铵(分析纯,上海阿拉丁生化科技股份有限公司);Co(NO3)2·6H2O(分析纯,上海麦克林生化科技有限公司);2-甲基咪唑(分析纯,上海阿拉丁生化科技股份有限公司);硫酸(分析纯,烟台市双双化工有限公司);甲醇(分析纯,烟台市双双化工有限公司);蒸馏水。
钴基活化剂的制备:利用球磨机将Co(NO3)2·6H2O与2-甲基咪唑按照质量比3∶7的比例混合,所得混合物置于氩气氛围下于920 ℃高温煅烧2 h得黑色粉末样品,先用10%的硫酸溶液清洗样品表面附着的含钴化合物,之后用甲醇洗涤过滤,并水洗至中性,即可制得表面富含碳纳米管的钴基活化剂,其微观形貌如图1(a)所示;经X-射线光电子能谱测定(图1(b)),并结合有关文献[13],可得所制钴基活化剂中钴元素主要以四氧化三钴(Co3O4)形式被负载于多孔碳表面,为简化表述,本文采用Co-NCP表示所制的非均相钴基活化剂。

图1 钴基活化剂Co-NCP的微观形貌和所含钴元素的 Co 2p X-射线光电子能谱
Fig.1 Scanning electron microscopy (SEM) image and Co 2p X-ray photoelectron spectrum (XPS) of Co-NCP activator
1.2.1 实验装置
本文采用的加压浸泡系统由自主研发的磁力高压反应釜和高压气瓶构成,如图2所示。该系统具有良好的气密性,可以实现不同温度、不同压力的浸泡环境。

图2 加压浸泡系统
Fig.2 Pressure soaking system 1—气瓶;2—温度显示;3—转速显示;4—进气阀门;5—压力表; 6—出气阀门;7—温度传感器;8—加热开关;9—电源开关; 10—磁子;11—煤样
1.2.2 实验方法
为研究Co-NCP/APS体系对煤样的增透效果,主要进行以下7个方面的实验:新鲜煤块切片、破碎,将粒径6 mm的颗粒煤样在不同溶液中浸泡72 h,60 ℃干燥2 d,在扫描电子显微镜(SEM,SU8220型冷场发射扫描电镜)下观察改性前后煤样表面形貌的变化;选取粒径为2~3 mm的煤粒,干燥后采用磁力高压反应釜对煤样进行不同溶液处理,处理时间均为72 h,将煤样烘干至恒重,进行压汞实验测试,分析改性前后煤样的孔隙结构与孔隙连通性等信息,压汞实验采用型号为AutoPore IV 9510的高性能全自动压汞仪,仪器工作压力为0~414 MPa,孔径测量范围为3 nm~1 000 mm;筛选60~80目的长焰煤、焦煤、无烟煤密封保存,各煤样在30 ℃条件下采用不同溶液加压浸泡72 h,采用等温吸附实验和瓦斯放散初速度实验研究改性前后不同变质程度煤样亲甲烷能力的变化;将煤样分别加工成底面直径5 cm,厚度1 cm的圆柱形煤片,用砂纸打磨煤片至表面光滑,使用蒸馏水为润湿液,利用Kruss DSA25光学测量仪对不同溶液处理的煤样表面进行接触角测量,每个煤样测5组,取平均值为最终接触角θ,研究Co-NCP/APS体系对煤样润湿性的影响;将煤样制作成φ50 mm×50 mm规格的煤柱,采用河南理工大学“三轴瓦斯渗流实验系统”测量煤样的渗透率,研究Co-NCP/APS体系对不同变质程度煤样的增透效果,测试时仪器设定条件为:轴压1 MPa、围压1 MPa,气体压力0.3 MPa,每种煤样用3个煤柱进行平行试验以减少实验误差;对处理煤样后的滤清液用Tekmar Dohrmann Apollo 9000型TOC分析仪进行总有机碳(TOC)测试。
1.2.3 实验步骤
文中不同尺度煤样加工所涉及的设备主要有:立式钻床、球磨机、干燥箱等。为探究Co-NCP/APS体系对煤样的氧化增透效果进行了系列实验,样品编号见表2,煤样尺寸、形状及实验步骤如图3所示。
表2 不同溶液处理煤样的编号
Table 2 Number of coal samples treated with different solutions
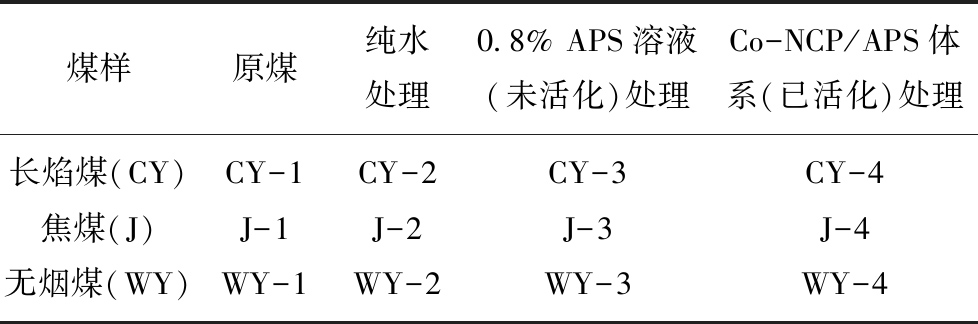
煤样原煤纯水处理0.8% APS溶液(未活化)处理Co-NCP/APS体系(已活化)处理长焰煤(CY)CY-1CY-2CY-3CY-4焦煤(J)J-1J-2J-3J-4无烟煤(WY)WY-1WY-2WY-3WY-4
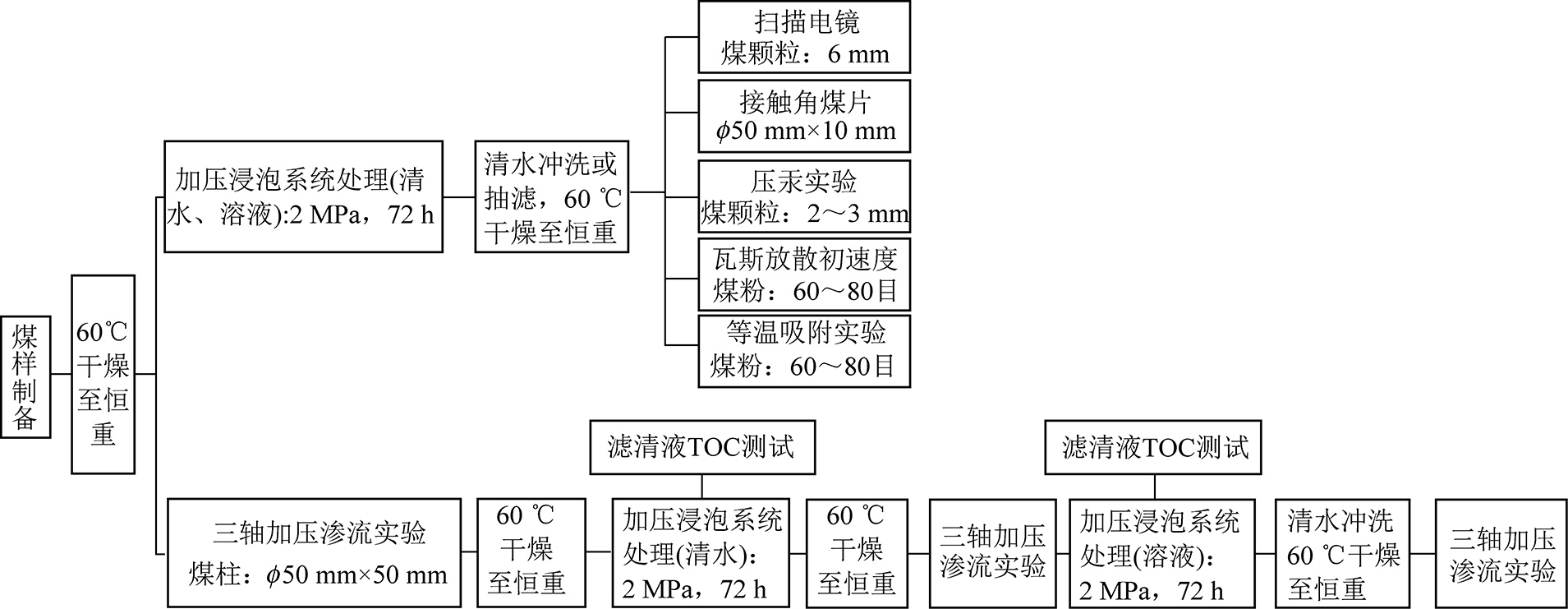
图3 实验步骤
Fig.3 Experimental procedure
瓦斯的主要成分甲烷是呈中心对称的非极性分子,而水分子是极性分子,对水分子有较好吸附性的物质则对甲烷吸附性较弱。通常用接触角θ对煤的润湿性进行表征:θ>90°说明煤表面呈疏水性,且θ越大表明煤表面疏水性越强,润湿难度越大;θ<90°说明煤表面呈亲水性,且θ越小表明煤表面亲水性越强[14-15]。因此,在对煤样进行化学氧化改性时,接触角θ变化越显著表明药剂对煤的氧化改性效果越好,改性后煤吸附瓦斯的倾向性越低。
由表3和图4可知,未活化的APS溶液能轻微氧化煤样,减小接触角,但效果不明显;在氧化体系中引入非均相钴基活化剂Co-NCP活化过硫酸铵则可大幅度提高APS氧化煤样的效果,显著减小接触角。由表4可知,温度对活化APS体系氧化煤表面有较大影响,对于长焰煤、焦煤与无烟煤,经Co-NCP/APS体系处理后的接触角均随温度的升高而显著降低;而且表4数据显示30 ℃的处理温度即可显著改善煤样润湿性,结合煤层实际地温条件,本文选择在30 ℃条件下对煤样进行APS溶液改性增透实验[16]。
表3 焦煤接触角
Table 3 Contact angles of coking coal
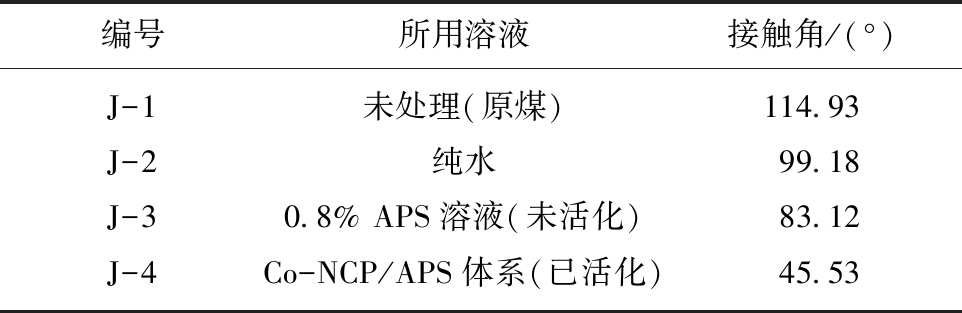
编号所用溶液接触角/(°)J-1未处理(原煤)114.93J-2纯水99.18J-30.8% APS溶液(未活化)83.12J-4Co-NCP/APS体系(已活化)45.53
注:温度条件为30 ℃。

图4 不同溶液处理焦煤的接触角
Fig.4 Contact angles of coking coal treated with different solutions
表4 不同温度条件下煤样接触角数据
Table 4 Contact angle data of coal samples under different temperature conditions (°)
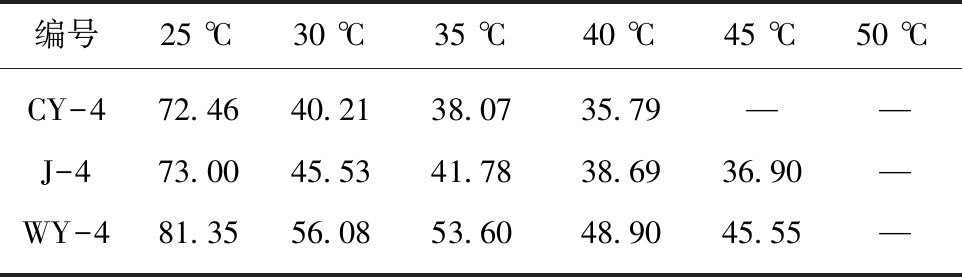
编号25 ℃30 ℃35 ℃40 ℃45 ℃50 ℃CY-472.4640.2138.0735.79——J-473.0045.5341.7838.6936.90—WY-481.3556.0853.6048.9045.55—
注:“—”表示煤表面被腐蚀严重无法进行接触角的测量。
对比不同变质程度煤样原煤及经清水、未活化APS溶液、活化APS溶液处理后颗粒煤的扫描电镜图发现,经清水、未活化APS溶液处理,煤样表面没有发生明显变化,而经活化APS溶液处理的煤样表面变得较为松散、粗糙,并且出现了大量新的裂隙与孔洞(图5);而且,结合原煤样与活化APS溶液处理后煤片表面照片可知,相同处理条件下长焰煤表面被溶蚀程度更高,表面变得更为粗糙,这归因于低阶煤芳香环聚合化程度低,煤结构中小分子相组分含量更高,煤体更易被活化的APS溶液氧化溶蚀[8,17-18]。
不同煤样的孔容与孔隙度见表5,相应的进-退汞曲线如图6所示。对比表5可知,经过Co-NCP/APS体系处理后3种煤样的孔隙度与孔容均得到不同程度的增加。长焰煤、焦煤、无烟煤的孔隙度增幅分别为43.50%,35.50%,12.54%,总孔容增幅分别为56.02%,45.54%,20.08%。在相同的实验条件下,低阶煤样的孔隙度与孔体积增幅更为显著,说明Co-NCP/APS体系处理煤样有利于疏通煤体瓦斯运移的通道,促进煤层气在煤体中的渗流。此外,从表5中数据可看出,改性后煤样的孔隙结构发生了改变,Co-NCP/APS处理后煤样的大、中孔孔容及占比明显增大,小、微孔孔容及占比均有所减小,Co-NCP/APS体系处理煤样能起到增孔与扩孔的作用[19-20]。对比Co-NCP/APS体系处理前后不同变质程度煤样的进汞-退汞曲线(图6)可知,未处理煤样的进汞曲线与退汞曲线基本重合,表明原煤样的孔隙连通性较差;改性处理后煤样的进汞-退汞曲线出现明显的“滞后环”,且变质程度越低的煤样进汞-退汞曲线开口越大,表明Co-NCP/APS改性处理可有效改善煤样的孔隙连通性,使堵塞孔或封闭孔转变为半开放型、开放型孔[21-22]。此外,煤样处理前后同一区间内的进汞增量显著增加,表明Co-NCP/APS改性可使煤中不同孔径阶段孔隙的排驱压力明显减小,孔隙的连通性得到改善,有利于瓦斯的流动[20]。即Co-NCP/APS体系处理煤样能起到“增孔”“扩孔”与“疏孔”的作用。
通过对不同溶液改性煤样的等温吸附常数及瓦斯放散初速度进行测定,分析煤样的亲甲烷能力变化。
如表6和图7所示,利用Langmuir方程对煤样的瓦斯吸附等温线进行拟合,其拟合相关系数均大于0.99。由图7可看出,与原煤样相比,经清水与未活化APS溶液处理后的煤样瓦斯吸附能力略有下降,而用Co-NCP活化的APS溶液处理后煤对瓦斯的吸附能力显著降低。由表6可知,经清水、未活化APS溶液、Co-NCP/APS体系处理后煤样的吸附常数a,b值均有不同程度的降低,但Co-NCP/APS体系的降幅最大。吸附常数a值表征煤样对瓦斯的极限吸附量、b值表征煤样吸附瓦斯的速率[23-24]。经由Co-NCP/APS体系处理的长焰煤、焦煤、无烟煤a值降幅分别为16.15%,10.21%,6.03%,b值降幅分别为44.97%,41.10%,28.32%,表明活化的APS溶液处理煤样对变质程度较低的长焰煤瓦斯吸附能力影响最大,减缓瓦斯释放与降低瓦斯释放速度效果最显著。

图5 改性前后煤片表面与颗粒煤表面形貌对比
Fig.5 Comparison of coal surface and surface morphology of granular coal before and after modification
表5 压汞实验测得的煤样孔容及孔隙度基本数据
Table 5 Obtained data of pore volume and porosity of coal samples measured by mercury injection experiments

编号孔隙度/%总孔容/(mL·g-1)大孔孔容/(mL·g-1)占比/%中孔孔容/(mL·g-1)占比/%小孔孔容/(mL·g-1)占比/%微孔孔容/(mL·g-1)占比/%CY-212.000.088 90.035 940.380.007 68.550.014 115.860.031 335.21CY-312.780.090 90.037 240.880.007 17.850.013 514.800.033 236.47CY-417.220.138 70.096 169.290.018 313.190.003 12.240.021 215.28J-212.310.100 80.067 867.260.002 62.580.009 49.330.021 020.83J-312.280.100 50.068 167.760.003 53.400.008 07.820.020 920.39J-416.680.146 70.113 977.640.004 43.000.008 15.520.020 313.84WY-220.330.302 30.227 975.390.005 21.720.021 87.210.047 415.68WY-320.420.304 80.229 975.430.006 01.970.018 66.100.050 316.50WY-422.880.363 00.306 484.410.017 94.930.015 84.350.022 96.31

图6 煤样的进汞-退汞曲线
Fig.6 Mercury injection and withdrawal curves of coal samples

图7 不同溶液处理煤样的等温吸附实验数据Langmuir 方程拟合曲线
Fig.7 Langmuir equation fitting curves of isothermal adsorption experiment data of coal samples treated with different solutions
表6 煤样的等温吸附及瓦斯放散初速度数据
Table 6 Isothermal adsorption data and Δp of coal samples

样品编号Δp/Pa工业分析/%水分灰分挥发分吸附常数a/(m3·t-1)b/MPa-1R2CY-14933.3116.6144.3610.351.350.999 8CY-24213.9016.1744.3010.081.070.999 5CY-34193.8816.1344.2010.181.030.999 3CY-42514.5315.9238.158.680.740.999 9J-18000.7011.8328.6718.051.950.999 7J-27200.9311.5428.3418.011.360.999 9J-37170.9511.6828.5317.831.440.998 8J-45331.1511.6225.1816.211.150.999 9WY-13061.3511.578.8733.782.280.999 9WY-22411.3211.238.6733.562.040.999 9WY-32371.4011.488.3534.361.940.999 1WY-41421.7611.497.8531.751.630.999 6
煤样的瓦斯放散初速度(Δp)值是表征煤体放散瓦斯能力的重要物理量,是预测瓦斯突出的关键指标之一[25],是指3.5 g 60~80目煤样在常压下吸附瓦斯1.5 h后向固定真空空间释放时,用压差Δp(Pa)表示的最初10~60 s内释放出瓦斯量的指标。利用WT-1型瓦斯扩散速度测定仪测定原煤和各处理煤样Δp值,由表6可知,与原煤相比,经Co-NCP/APS体系处理后3种煤样的瓦斯放散初速度分别降低了49.19%,33.33%,12.55%;而且变质程度越低的煤样,其Δp降幅越大,这主要是由于改性后煤样表面的物理化学性质发生了变化,导致其亲甲烷能力降低[20],这将直接导致在Δp测试中吸附瓦斯阶段煤样吸附瓦斯量减少,进而导致在向真空空间释放阶段释放出的瓦斯量减少,最终造成处理后煤样的Δp值降低。结合压汞实验及工业分析数据可知,改性后煤样的孔隙度增大,大、中孔的孔容占比增加,小、微孔孔容占比减少,降低了煤样对瓦斯的赋存能力;且改性后煤样的挥发分含量有所降低,表明活化的过硫酸铵通过氧化煤中小分子相组分,能削弱有机小分子与煤主体结构或煤孔隙间的作用,使其更易于析出、溶解于改性溶液中,并有利于疏通被小分子有机物占据或封堵的孔道[26],改善瓦斯在煤体中的流动性。
采用河南理工大学“三轴瓦斯渗流实验系统”测量煤样的渗透率,该系统包括高压气瓶、煤样夹持装置、恒温装置、流量采集装置、围压和轴压加载装置等,具体装置如图8所示。先对煤样的原始渗透率进行测试,按照前述方法用清水对煤样进行加压浸泡处理,完成渗透率的测试;测试完成后将煤样放入干燥箱,干燥至恒重后取出,分别用未活化的APS溶液或Co-NCP/APS体系对煤柱进行加压浸泡处理,测定其渗透率,测试结果见表7。

图8 三轴瓦斯渗流实验系统
Fig.8 Three-axis gas seepage experiment system 1—煤样夹持器;2—煤柱;3,4—手动加压泵;5—高压气罐; 6—恒温装置;7—集气瓶;8—减压阀;9,10,11,12,13—压力表; 14,15,16,17,18—阀门
表7 不同溶液处理后煤样及原煤样的渗透率实验结果
Table 7 Permeability test results of treated coal samples with different solutions as well as raw coal samples

煤样分类原煤渗透率/10-15m2(CY-1,J-1,WY-1)清水处理(CY-2,J-2,WY-2)渗透率/10-15m2对原煤增幅/%平均增幅/%普通APS溶液处理(CY-3,J-3,WY-3)渗透率/10-15m2对原煤增幅/%平均增幅/%活化APS溶液处理(CY-4,J-4,WY-4)渗透率/10-15m2对原煤增幅/%平均增幅/%对清水处理增幅/%平均增幅/%0.780 51.288 165.041.409 580.59———长焰煤0.378 00.611 161.6661.820.645 370.7171.50—————0.025 00.039 758.750.040 863.20———0.030 60.051 467.98——0.237 7676.80608.82长焰煤0.886 31.378 655.5558.80———6.254 2605.65620.15550.10561.360.286 70.438 352.88——1.943 9578.03525.151.224 31.856 751.651.903 555.48———焦煤0.396 10.620 756.7051.560.638 461.1763.04—————0.036 70.053 746.320.063 372.48———1.161 31.684 245.03——7.907 1580.88535.85焦煤0.645 10.992 553.8547.28———4.172 1546.74541.02492.89493.710.047 20.067 542.96——0.281 0495.34452.381.178 81.732 847.001.762 149.48———无烟煤0.431 20.606 140.5542.150.653 651.5848.35—————0.069 80.096 938.890.100 543.98———0.078 90.115 446.22——0.360 8357.29311.07无烟煤1.375 91.923 639.8140.41———6.062 2340.60334.42300.79293.990.641 80.867 835.21——2.601 3305.31270.10
由表7可知,长焰煤、焦煤、无烟煤采用未活化的APS溶液与清水进行加压浸泡处理,可使煤柱的渗透率产生较小幅度的增加,且两者对渗透率的增幅相近。而经由Co-NCP/APS体系对煤样进行改性处理,能使煤样渗透率产生较大幅度的提高。长焰煤、焦煤、无烟煤的渗透率相较原煤增幅率分别为:620.15%,541.02%,334.42%,Co-NCP/APS溶液对煤柱的增透效果明显优于未活化的APS溶液或清水。同时,相较于清水处理的煤样,Co-NCP/APS体系处理后煤样渗透率增幅分别为:561.36%,493.71%,293.99%,而未活化的APS溶液处理后煤样的渗透率相比于清水处理渗透率的增幅仅为:9.68%,11.49%,6.20%,说明非均相Co-NCP能有效活化过硫酸铵[12],使Co-NCP/APS体系具有较强的氧化性能,从而对煤样改性处理起到更优的增透效果。对比不同变质程度煤样的渗透率发现,渗透率增幅随着煤阶的升高而降低,说明Co-NCP/APS体系对变质程度较低的煤增透效果更好。
煤是有机大分子相与小分子相组成的复杂混合物[27-28],其中的有机小分子相主要包括一些脂肪烃和多种苯系、萘系等芳香烃类化合物[28-29];而且,不同地区或变质程度煤样所含有机小分子相的组成与比例存在较大差异[27]。季淮君等[30]采用溶剂萃取法研究了有机小分子相组分对煤样孔隙结构、瓦斯解吸速率与渗透特性的影响,结果表明通过四氢呋喃等溶剂萃取可移除充填或镶嵌在煤孔隙结构中的有机小分子相物质,进而改变煤的孔隙结构,一定程度上起到增孔、扩孔的作用,降低煤对甲烷的吸附能力;同时,减小煤粒表面瓦斯传质与扩散阻力,提高煤样的渗透率。
溶剂萃取虽可溶解煤中部分有机小分子相组分,但该方法需使用大量的化学试剂[31]。活化过硫酸铵产生的硫酸根自由基![]() 具有长的半衰期与高的氧化电势(2.5~3.1 V)[11],可快速氧化、分解石油烃、苯类、酚类、多环芳烃等有机污染物[10,32-33]。以构建的Co-NCP/APS体系处理煤样,Co-NCP所含负载型Co3O4经由反应
具有长的半衰期与高的氧化电势(2.5~3.1 V)[11],可快速氧化、分解石油烃、苯类、酚类、多环芳烃等有机污染物[10,32-33]。以构建的Co-NCP/APS体系处理煤样,Co-NCP所含负载型Co3O4经由反应![]() 能有效活化APS[34],原位产生的
能有效活化APS[34],原位产生的![]() 可氧化、溶蚀煤样孔隙结构中的有机小分子相组分,同时改变煤孔隙的表面性质。为进一步探究活化过硫酸铵对煤样改性增透的作用机理,对处理渗透率测试用煤柱的滤清液进行了总有机碳测试(Tekmar Dohrmann Apollo 9000型TOC分析仪)[35]。通过测定处理煤样所得滤液中的总有机碳含量可为
可氧化、溶蚀煤样孔隙结构中的有机小分子相组分,同时改变煤孔隙的表面性质。为进一步探究活化过硫酸铵对煤样改性增透的作用机理,对处理渗透率测试用煤柱的滤清液进行了总有机碳测试(Tekmar Dohrmann Apollo 9000型TOC分析仪)[35]。通过测定处理煤样所得滤液中的总有机碳含量可为![]() 氧化并溶蚀煤中小分子相物质提供实验证据,从图9可看出,对于长焰煤,清水或单一钴基活化剂溶液处理煤样所得滤液中TOC含量仅为56 mg/L与59 mg/L,未活化APS水溶液处理煤样后的滤清液中TOC含量为103 mg/L,被Co-NCP活化的APS溶液处理煤样所得滤液中TOC含量高达558 mg/L,这主要是由于未经活化的APS溶液中大量存在的是过硫酸根离子
氧化并溶蚀煤中小分子相物质提供实验证据,从图9可看出,对于长焰煤,清水或单一钴基活化剂溶液处理煤样所得滤液中TOC含量仅为56 mg/L与59 mg/L,未活化APS水溶液处理煤样后的滤清液中TOC含量为103 mg/L,被Co-NCP活化的APS溶液处理煤样所得滤液中TOC含量高达558 mg/L,这主要是由于未经活化的APS溶液中大量存在的是过硫酸根离子![]() 单一
单一![]() 的氧化性能较弱,无法实现对煤样的表面改性与增透;而经Co-NCP活化的APS体系,存在大量的硫酸根自由基
的氧化性能较弱,无法实现对煤样的表面改性与增透;而经Co-NCP活化的APS体系,存在大量的硫酸根自由基![]() 这一自由基具有强的氧化性能[10,31-33],可有效氧化、溶蚀煤中有机小分子相物质。对于焦煤与无烟煤,处理溶液组成对所得滤清液中TOC含量的影响也呈现出类似的情况。同时,由表6中的煤样工业分析数据可知,Co-NCP/APS体系处理后煤样的挥发分有所降低,这进一步表明活化的APS可使煤中有机小分子相发生部分溶出。
这一自由基具有强的氧化性能[10,31-33],可有效氧化、溶蚀煤中有机小分子相物质。对于焦煤与无烟煤,处理溶液组成对所得滤清液中TOC含量的影响也呈现出类似的情况。同时,由表6中的煤样工业分析数据可知,Co-NCP/APS体系处理后煤样的挥发分有所降低,这进一步表明活化的APS可使煤中有机小分子相发生部分溶出。

图9 不同溶液处理煤柱后所得滤清液的总有机碳(TOC)
Fig.9 Total organic carbon (TOC) of obtained filtration supern- atant after the treatment of coal column with different solutions
Co-NCP/APS体系通过氧化、溶蚀煤体孔隙中的小分子相物质,疏通了被小分子有机物占据或封堵的孔道,增大了小孔孔容,减小了小孔在整体孔径分布中所占的比例,起到“增孔”、“扩孔”、“疏孔”的作用;同时,APS活化产生的![]() 能原位氧化煤样表面,增加煤表面润湿性,降低煤体的亲甲烷能力。即通过Co-NCP/APS体系处理煤样,在增强孔隙连通性的同时,可降低煤体对瓦斯的赋存能力,并提升煤体的渗透性。
能原位氧化煤样表面,增加煤表面润湿性,降低煤体的亲甲烷能力。即通过Co-NCP/APS体系处理煤样,在增强孔隙连通性的同时,可降低煤体对瓦斯的赋存能力,并提升煤体的渗透性。
(1)采用自制的非均相钴基活化剂Co-NCP活化过硫酸铵,30 ℃时即可大幅度提高APS氧化改性煤样的效果,显著降低煤表面接触角θ与亲甲烷能力;改性后长焰煤、焦煤、无烟煤a值降幅分别为16.15%,10.21%,6.03%,b值降幅分别为44.97%,41.10%,28.32%,活化的APS溶液可有效降低煤体赋存瓦斯的能力。
(2)活化的过硫酸铵溶液可对不同变质程度煤表面实现氧化溶蚀,改性后煤表面变得更为粗糙,出现了大量新的裂隙和孔洞,且变质程度越低的煤样被氧化溶蚀效果越明显。
(3)经由Co-NCP/APS体系处理,煤中小分子相被部分氧化溶出,使得煤孔隙结构发生变化,中大孔孔容及占比明显增大,微小孔孔容及占比均有所减小;而且,有利于疏通被小分子物质占据或封堵的孔道,使煤孔隙连通性变好,即Co-NCP/APS改性起到“增孔”、“扩孔”、“疏孔”的作用,长焰煤、焦煤、无烟煤的渗透率平均增幅分别为620.15%,541.02%,334.42%。
(4)Co-NCP/APS体系对变质程度较低的煤样改性增透效果更为显著,其机理为过硫酸铵被Co-NCP活化产生大量的强氧化性![]() 氧化溶蚀煤中的小分子相物质。
氧化溶蚀煤中的小分子相物质。
[1] FLORES R M.Coalbed methane:From hazard to resource[J].International Journal of Coal Geology,1998,35(1-4):3-26.
[2] 张群,冯三利,杨锡禄.试论我国煤层气的基本储层特点及开发策略[J].煤炭学报,2001,26(3):230-235.
ZHANG Qun,FENG Sanli,YANG Xilu.Basic reservoir characteristics and development strategy of coalbed methane resource in China[J].Journal of China Coal Society,2001,26(3):230-235.
[3] 翟成,李贤忠,李全贵.煤层脉动水力压裂卸压增透技术研究与应用[J].煤炭学报,2011,36(12):1996-2001.
ZHAI Cheng,LI Xianzhong,LI Quangui.Research and application of coal seam pulse hydraulic fracturing technology[J].Journal of China Coal Society,2011,36(12):1996-2001.
[4] 李廷春,张浩,张治高,等.综采工作面过大落差断层深孔预裂爆破技术[J].煤炭学报,2019,44(1):199-209.
LI Tingchun,ZHANG Hao,ZHANG Zhigao,et al.Deep hole pre-splitting blasting technology when fully mechanized coal mining face passes through high drop fault[J].Journal of China Coal Society,2019,44(1):199-209.
[5] 马铁华,崔春生,肖文聪.煤层气井高能气体压裂器性能测试系统的研究[J].煤炭学报,2014,39(9):1857-1861.
MA Tiehua,CUI Chunsheng,XIAO Wencong.Experimental study on the performance test system of high energy gas fracturing apparatus in coal-bed methane extraction[J].Journal of China Coal Society,2014,39(9):1857-1861.
[6] 郭红玉,苏现波,陈俊辉,等.二氧化氯对煤储层的化学增透实验研究[J].煤炭学报,2013,38(4):633-636.
GUO Hongyu,SU Xianbo,CHEN Junhui,et al.Experimental study on chemical enhanced penetration of coal reservoir by chlorine dioxide[J].Journal of China Coal Society,2013,38(4):633-636.
[7] 李胜,罗明坤,范超军,等.基于核磁共振和低温氮吸附的煤层酸化增透效果定量表征[J].煤炭学报,2017,42(7):1748-1756.
LI Sheng,LUO Mingkun,FAN Chaojun,et al.Quantitative characterization of the effect of acidification in coals by NMR and low-temperature nitrogen adsorption[J].Journal of China Coal Society,2017,42(7):1748-1756.
[8] 倪小明,李全中,王延斌,等.多组分酸对不同煤阶煤储层化学增透实验研究[J].煤炭学报,2014,39(S2):436-440.
NI Xiaoming,LI Quanzhong,WANG Yanbin,et al.Experimental study on chemical permeability improvement of different rank coal reservoirs using multi-component acid[J].Journal of China Coal Society,2014,39(S2):436-440.
[9] 吴磊.水基压裂液低温破胶机理与发展应用[J].精细石油化工进展,2018,19(3):13-20.
WU Lei.Low temperature gel breaking mechanism of water-base fracturing fluid and its development and application[J].Advances in Fine Pet Rochemicals,2018,19(3):13-20.
[10] 杨德敏,夏宏,程方平.过硫酸盐氧化修复有机污染土壤的研究现状[J].油气田环境保护,2019,29(1):29-32.
YANG Demin,XIA Hong,CHENG Fangping.Research status of persulfate oxidation remediation of organic polluted soil[J].Environmental Protection of Oil & Gas Fields,2019,29(1):29-32.
[11] 杨世迎,陈友媛,胥慧真,等.过硫酸盐活化高级氧化新技术[J].化学进展,2008,20(9):1433-1438.
YANG Shiying,CHEN Youyuan,XU Huizhen,et al.A novel advanced oxidation technology based on activated persulfate[J].Progress in Chemistry,2008,20(9):1433-1438.
[12] WU D M,YE P,WANG M Y,et al.Cobalt nanoparticles encapsulated in nitrogen-rich carbon nanotubes as efficient catalysts for organic pollutants degradation via sulfite activation[J].Journal of Hazardous Materials,2018,352:148-156.
[13] KHAN M A N,KLU P K,WANG C H,et al.Metal-organic framework-derived hollow Co3O4/carbon as efficient catalyst for peroxymonosulfate activation[J].Chemical Engineering Journal,2019,363:234-246.
[14] 郑梦浩,王兆丰,岳基伟,等.接触角测量中型煤与原煤的适用性研究[J].中国安全生产科学技术,2018,14(8):129-133.
ZHENG Menghao,WANG Zhaofeng,YUE Jiwei,et al.The applicability of the antennae to measure medium coal and raw coal[J].Journal of Safety Science and Technology,2018,14(8):129-133.
[15] MURSITO A T,HIRAJIMA T,LISTIYOWATI L N,et al.Surface physicochemical properties of semi-anthracitic coal from Painan-Sumatra during air oxidation[J].International Journal of Coal Science & Technology,2018,5(2):156-166.
[16] 徐传升.丁集矿地温随深度变化趋势及防治措施[J].煤炭技术,2014,33(9):321-322.
XU Chuansheng.Dingji mine temperature changes with depth trends and prevention measures[J].Coal Technology,2014,33(9):321-322.
[17] LI Yanling,HUANG Sheng,WU Youqing,et al.The roles of the low molecular weight compounds in the low-temperature pyrolysis of low-rank coal[J].Journal of the Energy Institute,2019,92(2):203-209.
[18] SUN W J,WANG N,CHU W,et al.The role of volatiles and coal structural variation in coal methane adsorption[J].Science Bulletin,2015,60(5):532-540.
[19] JI H J,LI Z H,PENG Y J,et al.Pore structures and methane sorption characteristics of coal after extraction with tetrahydrofuran[J].Journal of Natural Gas Science and Engineering,2014,19:287-294.
[20] 宋金星.煤储层表面改性增产机理及技术研究[D].焦作:河南理工大学,2016.
SONG Jinxing.Study on mechanism and technology of surface modification and increasing production of coal reservoir[D].Jiaozuo:Henan Polytechnic University,2016.
[21] 傅雪海,秦勇,韦重韬.煤层气地质学[M].徐州:中国矿业大学出版社,2007.
[22] YAN F Z,XU J,LIN B Q,et al.Changes in pore structure and permeability of anthracite coal before and after high-voltage electrical pulses treatment[J].Powder Technology,2019,343:560-567.
[23] 白建平,张典坤,杨建强,等.寺河3号煤甲烷吸附解吸热力学特征[J].煤炭学报,2014,39(9):1812-1819.
BAI Jianping,ZHANG Diankun,YANG Jianqiang,et al.Thermodynamic characteristics of adsorption-desorption of methane in coal seam 3 at Sihe Coal Mine[J].Journal of China Coal Society,2014,39(9):1812-1819.
[24] CHENG Y P,JIANG H N,ZHANG X L,et al.Effects of coal rank on physicochemical properties of coal and on methane adsorption[J].International Journal of Coal Science & Technology,2017,4(2):129-146.
[25] 张小东,李朋朋,张硕.不同煤体结构煤的瓦斯放散特征及其影响机理[J].煤炭科学技术,2016,44(9):93-98.
ZHANG Xiaodong,LI Pengpeng,ZHANG Shuo.Gas emission features of coals with different coalbody structure and their influencing mechanism[J].Coal Science and Technology,2016,44(9):93-98.
[26] 张登峰,贾帅秋,伦增珉,等.煤体中小分子有机物赋存规律及其对煤体理化性质影响的研究进展[J].安全与环境学报,2018,18(6):2369-2378.
ZHANG Dengfeng,JIA Shuaiqiu,LUN Zengmin,et al.Inherent regularity of the minor organic molecules in coals and the research prospect with their physical-chemical properties[J].Journal of Safety and Environment,2018,18(6):2369-2378.
[27] MARZEC A.Towards an understanding of the coal structure:A review[J].Fuel Processing Technology,2002,77:25-32.
[28] ZONG Y,ZONG Z M,DING M J,et al.Separation and analysis of organic compounds in an Erdos coal[J].Fuel,2009,88(3):469-474.
[29] 季淮君,李增华,彭英健,等.煤的溶剂萃取物成分及对煤吸附甲烷特性影响[J].煤炭学报,2015,40(4):856-862.
JI Huaijun,LI Zenghua,PENG Yingjian,et al.Analysis of extracts and effects of them on methane adsorption characteristics of coal[J].Journal of China Coal Society,2015,40(4):856-862.
[30] 季淮君,李增华,杨永良,等.中变质煤小分子相对煤的吸附及渗流特性影响[J].燃料化学学报,2015,43(3):281-288.
JI Huaijun,LI Zenghua,YANG Yongliang,et al.Effect of small organic molecule in mid-metamorphism coal on gas adsorption and flow characteristics[J].Journal of Fuel Chemistry and Technology,2015,43(3):281-288.
[31] DHAWAN H,UPADHYAYULA S,SHARMA D K.Design of experiments to optimize the extraction parameters of a power grade Indian coal[J].International Journal of Coal Science & Technology,2018,5(4):417-429.
[32] FERREIRA I D,PRIETO T,FREITAS J G,et al.Natural persulfate activation for anthracene remediation in tropical environments[J].Water Air and Soil Pollution,2017,228(4):146-156.
[33] RANC B,FAURE P,CROZE V,et al.Selection of oxidant doses for in situ chemical oxidation of soils contaminated by polycyclic aromatic hydrocarbons (PAHs):A review[J].Journal of Hazardous Materials,2016,312:280-297.
[34] GUAN R P,YUAN X Z,WU Z B,et al.Efficient degradation of tetracycline by heterogeneous cobalt oxide/cerium oxide composites mediated with persulfate[J].Separation and Purification Technology,2019,212:223-232.
[35] YANG J,WANG X H,DAI J,et al.Efficient visible-light-driven photocatalytic degradation with Bi2O3 coupling silica doped TiO2[J].Industrial & Engineering Chemistry Research,2014,53(32):12575-12586.