
移动阅读
煤与煤系气地质与勘查

移动阅读
LIU Jingjing,DAI Shifeng,SPIRO Baruch F,et al. Characteristics of mineral matter in the lignite from Mile Basin,Eastern Yunnan:New evidence for the seawater input during the peat accumulation process into the inland basin[J]. Journal of China Coal Society,2021,46(12):3948-3961.
在沉积过程中受到海水影响的煤通常含有高含量的硫。继WHITE和THIESSEN[1]在研究美国Illinois煤盆地时,首次发现海水与沉积环境之间的关系后,已有很多学者对海相成因的煤进行了研究[1-6]。WILLIAMS和KEITH[7]在研究Lower Kittanning煤时提出,上覆海相地层的煤通常比正常的煤含有较高含量的硫,并且煤中硫的含量在很大程度上取决于受海水影响的程度[2,8-9]。例如,内蒙古乌达煤田的太原组的9号和10号煤,在其泥炭堆积时受到了海水的影响,全硫质量分数分别为3.46%和3.42%,受海水影响中等的12号和13号煤层,全硫质量分数分别为2.29%和0.88%[5]。煤中高质量分数的硫会导致一系列的环境问题。在煤燃烧过程中,煤中的硫有可能随着烟气的冷却形成SO3,然后转变为H2SO4,造成对燃烧炉的腐蚀[10]。同时,燃烧炉中释放的硫氧化物也是酸雨的主要来源[2]。煤中硫的主要载体矿物(黄铁矿)通常也富含其他有害元素砷(As)和汞(Hg)等[11],在煤的利用过程中也会对环境和人体健康造成危害。另一方面,海相环境中形成的高硫煤也可能富集战略性关键元素,如铀(U)、钒(V)和硒(Se),特别是稀土元素(Rare Earth Elements和Yittrium,REY)[12-13],这些高度富集关键元素的煤可作为这些元素提取的潜在来源。基于上述2个方面,海相成因的高硫煤已在世界范围内引起学者的关注[2-6]。
中国西南地区,如广西合山、贵州贵定和紫云、云南砚山,晚二叠世海相碳酸盐岩层中沉积形成的煤通常称为超高有机硫煤(有机硫质量分数4%~11%),煤中的有机硫主要来源于海底喷流[12,14],海水的入侵不是主导因素[15-16]。受热液影响而导致煤中硫的富集,在其他地区也有报道。例如,TURNER 和RICHARDSON[17]在研究英格兰Northumberland煤田Westphalian A 和B煤时,发现煤中部分次生硫主要来源于富硫的热液,而该热液的供给源远离海水影响的区域。
成煤过程中受海水影响的煤层,通常具有特定的地质和地球化学特征。CHOU[9]提出,一般情况下,海相泥炭中黄铁矿硫平均占全硫的14%,比淡水中形成的煤层中黄铁矿质量分数高出一个数量级。莓球状黄铁矿通常形成于海相泥炭的早期成岩阶段[18-19]。我国西南地区晚二叠世的高硫煤或超高有机硫煤一般有海相碳酸盐顶板或沉积于局限碳酸盐台地[12,14-16,20-21]。煤中的有孔虫及腕足动物碎片、腹足类、介形虫和微生物化石的富集也指示了海相沉积环境[22]。GOODARZI和SWAINE[23]在研究澳大利亚和加拿大煤时,提出用B元素的质量分数评估受海水的影响程度:海相沉积的煤中B的质量分数>110×10-6,尽管其质量分数有时也受其他因素(如热液流体)的影响。另外,一些对沉积环境敏感的微量元素的质量分数以及它们的比值也可用来判别海水或淡水沉积环境。例如,受海水影响的煤中黄铁矿经常富集As,Se,Hg,Tl,Pb,Mo,Cd,Ni,Sb和Cu等元素[24]。SPIRO等[25]和DAI等[26]认为可以通过煤中同生的并且是海水来源的Sr的同位素,判断泥炭的堆积环境。
过去的研究通常认为,沉积于海相环境的煤具有海相碳酸盐顶板[14-16, 20-21]。但是,对于陆相环境中形成的煤或无海相碳酸盐顶板的煤,很难考虑到其在泥炭堆积期受到海水输入的影响。因此,通过分析煤的地球化学和矿物学特征,可以为此类型煤的形成提供是否受到海水影响的证据,并为煤盆地的区域构造演化历史提供煤地质学依据。本文的研究区为滇东新近纪弥勒盆地,长期被认为沉积于陆相沉积环境[27]。在对该盆地煤中锶同位素的研究基础上[25],笔者通过分析弥勒盆地中新世煤的元素地球化学和矿物学特征,发现了海水入侵的新证据,以期进一步揭示该盆地泥炭堆积时所经历的地质过程。
云南位于印度板块之东,扬子地块之西南,同时又处于特提斯构造域与环太平洋构造域的交接复合部位。因此,云南区域构造域与印度板块、扬子地块和太平洋板块的活动状态以及3者之间的相互作用密切相关[27]。本次研究区为云南省弥勒县(图1,修改自SCHOENBOHM等[28])境内的山心村煤矿,北距省会昆明143 km,南距红河州府蒙自148 km,隶属于弥勒盆地南端的跨竹矿区。山心村煤矿东西平均宽约1 km,南北长约0.78 km,面积约0.78 km2。矿区含煤地层为中新世小龙潭组,厚度为40~150 m,平均厚度为100 m,主要由泥岩、砂质泥岩,以及少量的粉砂岩、炭质泥岩、煤层和非海相的碳酸盐组成。小龙潭组下伏地层为始新统—渐新统木花果组,其不整合覆于中三叠世个旧组之上,厚度为150~530 m,上部和底部为棕红色砂岩,中部为深灰色微现暗红色厚层状砾岩,钙质胶结,与下伏地层呈不整合接触;个旧组地层厚度为300~2 500 m,为浅海碳酸盐岩相沉积,主要由灰岩及少量白云质灰岩和白云岩组成,岩溶发育,为盆地矿坑充水的主要含水层。小龙潭组上覆地层为上新世师宗渡组,平均厚度为156 m,主要由砂砾岩、粉砂岩和泥岩组成。第四系沉积厚度为0~15 m,主要由黄褐色黏土、细砂及砾石组成。

图1 哀牢山—红河断裂带及研究区地理位置(修改自SCHOENBOHM等[28])
Fig.1 Location of Ailao Shan-Red River Fault and the study area (Modified from SCHOENBOHM等[28])
本次采样区为弥勒盆地跨竹矿区,采集了含煤地层底部的M1煤层,共采集样品61件,包括顶板、底板和2个夹矸样品,以及57个煤样品(表1)。从煤层顶部至底部,依次命名为ML-1~ML-61,顶、底板和夹矸分别附后缀-R,-F,-P加以表示(表1)。
表1 滇东弥勒煤中的矿物(石膏和黄铁矿,灰基,%)、主要元素的氧化物(CaO,MgO,P2O5,煤基,%)和稀土元素的质量分数(μg/g),沉积环境敏感元素质量分数比(Ca/Mg,Sr/Ba,Th/U和V/Ni)以及锶同位素比
Table 1 Percentage of minerals (gypsum and pyrite),content of major element oxides (CaO,MgO and P2O5) and REY,the ratios between sedimentary environment-sensitive elements (Ca/Mg,Sr/Ba,Th/U和V/Ni),as well as the strontium isotopes of the Mile coals,Eastern Yunnan

样品石膏黄铁矿CaOMgOP2O5Ca/MgSr/BaTh/UV/Ni87Sr/86SrLaCePrNdSmML-1-R0.7531.680.1530.530.633.203.6043.5988.0810.5341.397.85ML-21.6410.970.1192.020.792.406.8319.3940.974.5218.423.85ML-32.2480.440.0126.031.232.320.760.708 5731.363.750.562.730.58ML-42.8750.530.0336.491.101.770.782.494.600.552.320.47ML-52.3220.450.0086.191.220.600.590.931.970.230.990.19ML-63.2860.600.0156.491.180.051.081.503.100.401.780.32ML-73.0080.620.0175.781.350.170.710.708 5910.641.540.200.960.21ML-82.6410.510.0406.191.211.510.852.303.670.401.670.27ML-92.3770.490.0245.761.273.881.002.224.100.481.990.36ML-102.4090.570.0305.011.171.216.116.4413.471.817.621.62ML-11bdl0.21.6321.140.0881.700.960.865.0026.0946.396.0723.574.45ML-12-P1.80.61.0061.860.1290.640.761.921.7536.6085.398.9434.286.22ML-1314.00.72.5370.790.0723.811.071.421.6813.1427.163.5314.683.02ML-1426.91.12.9780.560.1166.281.361.181.417.3511.911.385.501.07ML-152.7230.550.0215.941.330.902.391.993.790.502.100.42ML-163.3930.640.0586.321.191.651.390.708 5624.738.571.094.220.76ML-173.3540.590.0486.771.221.651.472.725.060.592.270.43ML-182.7830.540.0236.111.362.021.101.162.510.331.380.25ML-192.5330.450.0956.751.432.501.936.6810.861.214.480.72ML-202.6680.520.0196.111.352.221.501.332.730.361.490.30ML-212.3750.470.0105.971.421.960.961.192.420.301.210.25ML-223.2490.590.0136.581.261.770.562.424.470.572.390.49ML-233.7150.650.0136.771.324.341.121.452.930.381.580.31ML-2427.10.63.0400.530.0196.831.163.221.407.7214.251.736.481.06ML-253.5860.670.0156.421.462.121.132.043.730.502.100.45ML-262.9630.590.0185.981.492.001.721.743.390.461.900.37ML-272.7080.540.0606.021.671.961.322.323.960.512.030.39ML-282.6460.520.0666.081.742.671.381.773.210.361.560.34ML-292.6270.510.0166.181.532.831.120.708 4021.643.230.391.640.34ML-302.4760.520.0105.721.703.311.011.553.070.391.580.30ML-312.4190.510.0195.71.730.611.711.983.610.421.790.35ML-321.2310.280.0055.241.851.640.870.992.010.240.910.15ML-332.5080.530.0055.631.701.021.091.452.920.331.390.25ML-342.7220.560.0045.821.72nd0.680.708 4800.801.720.180.850.13ML-353.6540.730.0125.971.610.192.411.653.650.472.080.48ML-3615.90.82.8890.510.3646.712.151.991.2516.5532.073.2911.992.01ML-373.6310.920.1054.711.971.740.735.4510.221.214.610.83ML-383.5730.670.2236.352.081.241.950.708 4864.468.831.003.900.81
续表

样品石膏黄铁矿CaOMgOP2O5Ca/MgSr/BaTh/UV/Ni87Sr/86SrLaCePrNdSmML-3910.5bdl3.2190.530.5897.292.322.462.7211.2223.392.7110.271.73ML-402.8790.620.0255.51.540.682.893.617.230.913.850.82ML-4123.10.52.8050.790.0284.231.511.703.40np10.8023.363.1413.463.00ML-423.4620.700.0575.901.671.081.956.4113.481.566.431.27ML-433.0640.640.0545.671.672.812.635.0110.111.234.880.95ML-4414.10.13.0880.710.0255.181.431.283.646.0312.151.495.911.20ML-454.6490.900.0206.161.650.291.600.708 4453.036.620.823.470.71ML-464.4500.940.0415.661.680.602.130.708 3585.3612.501.526.461.41ML-47-P1.1331.810.1490.750.862.131.7832.8567.677.3027.594.94ML-4851.79.64.7581.040.0335.441.580.741.590.708 3506.4614.981.908.091.80ML-4970.112.44.5690.920.0195.891.670.601.060.708 4182.635.530.642.790.53ML-5051.06.04.3420.890.0255.791.361.071.410.708 4024.669.311.104.320.90ML-5117.00.53.5210.850.0224.941.441.532.494.999.771.174.650.84ML-523.3830.750.0105.361.780.771.360.708 3801.553.240.391.670.35ML-533.7100.790.0115.601.681.620.708 4331.523.330.401.730.38ML-543.2570.680.0345.691.272.182.578.1615.711.937.471.48ML-553.2540.690.0155.631.630.582.331.704.090.542.320.53ML-563.1370.710.0315.271.590.983.405.0610.41.275.231.18ML-573.9002.310.0332.011.830.322.432.785.850.763.490.80ML-580.4180.350.0181.401.322.072.2113.3027.073.6114.893.28ML-592.5661.080.0392.831.081.283.5824.5747.725.9223.214.86ML-602.4801.120.0382.641.031.694.4222.3544.115.4922.204.76ML-61-F2.8bdl2.1001.380.0491.810.841.224.0128.0758.006.8126.585.25WA*2.9700.690.0605.261.581.431.860.708 4524.749.571.184.810.98样品EuGdTbDyYHoErTmYbLuY/HoLaN/La*NCeN/Ce*NGdN/Gd*NYN/Ho*NML-1-R1.948.141.096.3027.391.173.580.493.320.4823.411.151.001.190.85ML-21.014.320.613.8319.290.742.290.282.030.3026.071.311.131.170.95ML-30.170.680.090.533.740.100.310.030.250.0337.401.791.131.291.32ML-40.110.510.050.322.120.050.180.020.160.0242.401.511.081.491.47ML-50.050.210.020.160.870.020.0800.07043.501.331.091.691.76ML-60.100.360.040.291.310.040.150.010.140.0132.751.601.111.371.07ML-70.060.200.020.170.790.020.0800.08039.502.131.271.271.44ML-80.070.320.030.211.170.030.1300.120.0139.001.841.161.631.26ML-90.090.380.040.251.510.040.140.010.150.0137.751.461.081.501.25ML-100.411.640.231.325.770.230.700.080.650.0825.091.210.971.150.91ML-111.114.530.623.6315.670.692.180.282.080.3122.711.160.901.170.83ML-12-P1.486.170.794.3217.000.822.430.342.380.3320.731.071.111.210.75ML-130.723.260.442.4110.860.451.310.161.150.1724.131.210.991.210.88ML-140.261.200.140.854.460.150.450.060.440.0629.731.551.061.341.09ML-150.100.460.050.361.740.060.200.020.180.0229.001.330.991.391.10ML-160.180.880.100.622.760.110.370.040.350.0425.091.150.921.420.88ML-170.110.470.050.351.540.060.200.020.190.0225.671.211.001.390.97
续表

注:bdl为低于检测值。
样品EuGdTbDyYHoErTmYbLuY/HoLaN/La*NCeN/Ce*NGdN/Gd*NYN/Ho*NML-180.060.250.020.210.960.030.110 0.130 32.001.211.011.521.25ML-190.160.790.080.572.710.110.350.040.400.0524.641.331.001.440.93ML-200.060.320.020.210.990.030.130.010.130.0133.001.240.991.681.07ML-210.050.250.020.190.810.020.090.010.08040.501.221.021.631.33ML-220.130.520.060.411.740.070.230.020.210.0224.861.381.001.360.86ML-230.110.350.050.241.120.060.140.020.130.0218.671.261.001.190.72ML-240.231.200.150.934.800.190.650.090.690.1025.261.100.931.300.94ML-250.110.440.050.361.520.060.210.020.200.0225.331.340.961.370.89ML-260.100.400.040.311.380.060.180.020.180.0223.001.230.951.350.90ML-270.090.400.050.321.490.050.190.020.180.0229.801.350.961.331.00ML-280.070.310.030.231.120.040.130.010.140.0128.001.771.191.371.07ML-290.070.320.030.271.170.040.160.010.140.0129.251.401.081.371.01ML-300.070.360.030.251.130.040.130.010.110.0128.251.251.001.731.15ML-310.080.400.040.281.280.050.150.010.140.0125.601.601.121.530.97ML-320.030.170.010.130.740.020.0800.09037.001.050.961.971.23ML-330.060.270.020.180.980.020.1000.100.0149.001.451.141.651.55ML-340.040.1500.100.640.010.06bdl0.05064.002.191.412.872.55ML-350.120.470.050.361.690.060.210.020.200.0128.171.401.081.301.02ML-360.472.230.271.607.390.291.010.121.000.1325.481.181.081.310.91ML-370.210.880.100.622.970.110.390.040.380.0427.001.170.981.340.96ML-380.210.870.100.672.810.110.330.040.330.0425.551.191.041.300.95ML-390.422.010.221.356.190.240.730.090.750.1025.791.061.001.400.93ML-400.190.880.110.793.170.130.410.050.420.0524.381.371.051.230.89ML-410.763.080.422.459.860.451.340.171.210.1521.911.241.001.170.79ML-420.301.400.160.995.040.170.550.060.530.0629.651.311.101.341.06ML-430.221.040.140.843.610.150.480.060.450.0724.071.161.001.190.87ML-440.271.190.150.984.320.180.580.080.580.0724.001.160.991.210.88ML-450.190.760.090.542.780.090.300.030.290.0330.891.281.071.291.16ML-460.341.420.201.164.640.200.590.070.530.0723.201.241.091.140.85ML-47-P1.265.200.694.2218.890.832.640.362.530.3722.761.141.061.210.82ML-480.461.820.251.426.000.260.790.090.720.0923.081.201.051.180.84ML-490.140.590.060.432.240.070.240.020.200.0232.001.531.171.401.17ML-500.210.870.110.713.320.120.420.050.410.0527.671.181.011.240.99ML-510.200.880.110.703.110.120.440.050.430.0525.921.211.011.260.91ML-520.080.340.040.271.350.040.150.010.140.0133.751.451.121.381.33ML-530.090.380.040.281.480.040.160.010.140.0137.001.361.111.491.38ML-540.301.520.181.125.190.190.680.090.720.0927.321.130.961.300.97ML-550.130.550.060.432.110.070.230.020.210.0230.141.161.021.301.14ML-560.271.260.160.984.190.160.570.060.510.0626.191.251.041.260.96ML-570.190.830.110.693.630.110.360.040.320.0333.001.711.131.181.20ML-580.783.600.522.9313.400.551.630.231.590.2324.361.170.951.150.89ML-591.034.950.673.9418.560.742.290.312.250.3125.081.140.961.180.91ML-601.034.870.693.9617.980.742.260.302.230.3124.301.221.001.150.88ML-61-F1.105.280.724.0516.870.792.440.352.490.3621.351.121.011.170.78WA0.231.040.130.83.660.140.450.050.430.0529.281.331.051.371.06
按照ASTM Standards D3174-11[31]测定煤中的灰分,按照ASTM Standards D3177-02[32]测定煤中全硫的质量分数。煤中元素C,H,N的质量分数由元素分析仪(Elementar,Vario MACRO)测得。
用X射线荧光光谱仪(ARL ADVANT′XP+)测定煤的氧化物及岩石样品中的常量元素的氧化物(CaO, MgO和P2O5)的质量分数。用电感耦合等离子质谱仪(Thermo Fisher,X series II ICP-MS)测定样品中的微量元素(Sr,Ba,Th,U,V,Ni和稀土元素)的质量分数。待测样品的预处理过程包括:称样、加酸、微波、消解、赶酸、提取和定容。对于煤和岩石(顶、底板和夹矸)样品,每50 mg样品,分别添加5 mL 65% HNO3,2 mL 40% HF和2 mL 65% HNO3,5 mL 40% HF 作为消解试剂,ICP-MS测试方法的检出限不高于0.02 ng/mL。
用X射线粉末衍射仪,结合商用软件SiroquantTM,对煤中的矿物(黄铁矿)进行定性和定量。用X射线荧光光谱仪(ARL ADVANT′XP+)测定煤的氧化物及岩石样品中的常量元素的氧化物(CaO,MgO和P2O5)。用电感耦合等离子质谱仪(Thermo Fisher,X series II ICP-MS)测定样品中的微量元素(Sr,Ba,Th,U,V,Ni和稀土元素)质量分数。用带能谱的场发射扫描电镜(FEI QuantaTM 650 FEG)观察和分析煤中的矿物形态和显微结构,并分析样品中部分元素的分布特征。
在同位素比值87Sr/86Sr测试前,将已破碎至200目(0.075 mm)的煤粉末样置于超纯水中,充分搅拌以溶解煤中的石膏。过滤以分离固体物和石膏溶解液。采用Triton Plus质谱分析仪测试锶的同位素,锶的同位素质量分馏用87Sr/86Sr=8.375 209校正[33]。国际标准样品NBS987测试的平均值87Sr/86Sr=0.710 250±0.000 010。
由于有机质的影响,所研究的57个煤样中仅有11个煤样品的XRD分析结果理想(表1)。弥勒原煤中的矿物主要为高岭石、伊利石、石膏、黄铁矿和锐钛矿。在灰分高于40%的样品中有金红石和绿泥石。部分样品中含有少量纤磷钙铝石、绿泥石和方解石。石膏的质量分数变化范围较大(为1.80%~70.10%,灰基),均值约为30%,个别煤分层样品(ML-49)中质量分数高达70%(表1),远高于其他地区煤中石膏的质量分数[34-35]。下部煤分层中石膏质量分数比上部煤分层中的高。
扫描电子显微镜下可见石膏呈钟乳状(图2(a)),或与同生黄铁矿共生(图2(b)),或分布于有机质中(图2(c))。煤中的石膏主要有2种成因:① 若原煤中存在方解石,其可能与![]() 相互作用形成;②由孔隙水中的以非矿物态存在的Ca和
相互作用形成;②由孔隙水中的以非矿物态存在的Ca和![]() 和结合形成。弥勒煤中石膏的赋存形态,表明它的形成机制属于后者,是在泥炭层被压实失水过程中形成的。煤中同生的黄铁矿颗粒分布于石膏表面(图2(b)),表明黄铁矿的形成晚于石膏,进一步表明石膏为同生矿物。另外,部分黄铁矿也呈莓球状,分布于碎屑腐植体中,部分呈单个晶体颗粒,充填于有机质孔隙中(图2(c))。煤中石膏在煤低温灰化过程中可形成烧石膏(CaSO4·0.5H2O)或硬石膏(CaSO4)[36-37] 。
和结合形成。弥勒煤中石膏的赋存形态,表明它的形成机制属于后者,是在泥炭层被压实失水过程中形成的。煤中同生的黄铁矿颗粒分布于石膏表面(图2(b)),表明黄铁矿的形成晚于石膏,进一步表明石膏为同生矿物。另外,部分黄铁矿也呈莓球状,分布于碎屑腐植体中,部分呈单个晶体颗粒,充填于有机质孔隙中(图2(c))。煤中石膏在煤低温灰化过程中可形成烧石膏(CaSO4·0.5H2O)或硬石膏(CaSO4)[36-37] 。

图2 弥勒煤中石膏和黄铁矿的扫描电子显微镜背散射图像
Fig.2 SEM back scatter image of gypsum and pyrite in the coal from Mile Basin
3.2.1 常量和微量元素
研究区煤中硫的质量分数变化为0.19%~3.78%,均值为0.78%(干燥基)(表1)。高硫含量(>1%)的分层位于剖面的底部和上部。2个连续的煤分层ML-48和ML-49中全硫的质量分数分别为3.78%和3.60%。在这些样品中,有机硫和硫酸盐硫是硫的主要赋存形式,分别占全硫质量分数的40%~60%和25%~42%。
煤中部分常量元素氧化物、微量元素的含量以及锶同位素比值87Sr/86Sr见表1。CaO的质量分数为1.01%~4.76%,均值为2.97%(煤基),高于中国煤中CaO质量分数的均值(1.23%[38])。MgO的质量分数为0.28%~1.14%,均值为0.69%,高于中国煤中MgO质量分数的均值(0.22%[38])。
微量元素Sr的质量分数为61.73~1 011.28 μg/g,均值为204.4 μg/g,高于世界低阶煤中Sr的平均质量分数(120 μg/g[39])。Ba的质量分数均值为137.01 μg/g,与世界低阶煤中的均值(150 μg/g[39])相当。微量元素Th和U的质量分数均值分别为2.30,1.78 μg/g,均低于世界低阶煤的均值(分别为3.30,2.90 μg/g[39])。
3.2.2 稀土元素
稀土元素包括镧系元素(La,Ce,Pr,Nd,Sm,Eu,Gd,Tb,Dy,Ho,Er,Tm,Yb和Lu)和Y,共15个元素。按照SEREDIN和DAI[40]提出的稀土元素分类方法,将稀土元素分为轻稀土(LREY:La,Ce,Pr,Nd,Sm)、中稀土(MREY:Eu,Gd,Tb,Dy和Y)和重稀土(HREY:Ho,Er,Tm,Yb和Lu)。利用上地壳中稀土元素的质量分数对研究区样品中稀土元素的质量分数进行标准化后,将样品中稀土元素的富集类型划分为3种:轻稀土富集型(LaN/LuN>1)、中稀土富集型(LaN/SmN<1,GdN/LuN>1)和重稀土富集型(LaN/LuN<1)。据BAU等[41]和DAI等[42]提出的计算方法,对Ce,Eu,La,Gd和Y的异常按照式(1)~(4)计算。
(1)
(2)
(3)
(4)
其中,下标N为用上地壳的稀土元素含量进行标准化后的值;*为标准化后稀土元素含量的期望值,异常值大于1和小于1分别表示稀土元素的正异常和负异常,等于1表示无异常。
弥勒煤层剖面上部的分层以轻—中稀土富集型为主(图3(a)~(c));下部以中—重稀土富集型为主(图3(d)~(e))。煤中![]() 为0.90~1.41,均值为1.05(表1),显示微弱的正异常或无明显异常(图3)。煤层以及含煤地层中Ce的异常主要与沉积源区的母岩岩性、地下水、热液、海水和Fe-Mn氢氧化物的矿化有关[40,42]。其中,沉积源区的岩性是造成所研究样品中Ce异常的主要因素[43-44]。中国大部分煤通常具有微弱的Gd负异常特征[42]。但是在本次研究的弥勒煤中,Gd呈显著的正异常(均值为1.37,表1,图3)。造成Gd正异常的因素主要有海水、热液或其他水体的影响[42]。煤中
为0.90~1.41,均值为1.05(表1),显示微弱的正异常或无明显异常(图3)。煤层以及含煤地层中Ce的异常主要与沉积源区的母岩岩性、地下水、热液、海水和Fe-Mn氢氧化物的矿化有关[40,42]。其中,沉积源区的岩性是造成所研究样品中Ce异常的主要因素[43-44]。中国大部分煤通常具有微弱的Gd负异常特征[42]。但是在本次研究的弥勒煤中,Gd呈显著的正异常(均值为1.37,表1,图3)。造成Gd正异常的因素主要有海水、热液或其他水体的影响[42]。煤中![]() 和
和![]() 值均差异较大,变化范围分别在0.79~2.55和1.05~2.19,均值分别为1.08和1.33(表1),显示正异常特征。
值均差异较大,变化范围分别在0.79~2.55和1.05~2.19,均值分别为1.08和1.33(表1),显示正异常特征。

图3 弥勒煤及顶底板、夹矸中稀土元素的分布特征
Fig.3 REY distribution of the Mile coals,roof,floor and parting samples
3.2.3 石膏中锶同位素87Sr/86Sr特征
由于Sr与Ca具有相似的离子半径,石膏的矿物晶格中可能有部分Ca被Sr替换。因此,同沉积的自生石膏中Sr的同位素值87Sr/86Sr能够反映泥炭堆积时期溶液的性质。弥勒盆地13个煤样中石膏的87Sr/86Sr比值变化为0.708 350~0.708 591(表1),它在煤层剖面上的变化如图4(a) 所示。从煤层顶部至底部,可将该数据点依次分为A,B和C三组,并且整体上呈递减趋势(图4(a))。

图4 弥勒煤中石膏的87Sr/86Sr比值和早中新世浮游有孔虫的87Sr/86Sr比值(修改自HODELL等[85])
Fig.4 87Sr/86Sr values of gypsum through the Mile coal seam and the87Sr/86Sr values of planktonic foraminifera of Early Miocene(Modified from HODELL etc[85])
高含量的同生黄铁矿通常是受海水影响的煤的显著特征之一[1,3,7,45-50],虽然在煤层形成的不同阶段热液成因的黄铁矿也经常被发现。在不同海相沉积物中,莓球状黄铁矿的形成机制相似,均是由四方硫铁矿(FeS0.9)经硫复铁矿(Fe3S4)转变形成。而黄铁矿单个晶体颗粒则一般由FeS与聚硫化物作用形成[51-53]。在上述黄铁矿的2种形成机制中,海水中的硫酸盐是各作用过程中所需的H2S的主要来源。因此,弥勒煤中丰富的同生黄铁矿,表明在泥炭堆积过程中受到了海水的影响。
弥勒煤中夹矸和底板中石膏的质量分数均小于3%,远低于煤层中石膏的质量分数。沉积岩中的石膏为蒸发作用的产物,湖盆和海盆中的卤水在干旱的气候条件下,经蒸发和浓缩作用后结晶而成。煤中的石膏通常被认为是次生矿物[36-37]。SPIRO等[25]通过对弥勒煤中石膏赋存状态的研究,认为其为同生沉积,来源可能为海水,经泥炭失水、压实后结晶而形成。在底部的煤层中(ML-48,ML-49,ML-50),石膏的质量分数远高于顶部和中部煤层(表1),表明底部煤层受海水影响程度较大,表明随着泥炭的堆积,海水的输入逐渐减少。
除了上述黄铁矿外,煤中有机硫的性质能够为其沉积环境提供一定的信息[54]。成煤过程中海水的输入为泥炭提供了充足的硫酸盐作为硫源,因此,煤中丰富的黄铁矿和有机硫指示了成煤的海相沉积环境[3,7-9,55]。CHOU[55]在研究伊利诺伊盆地赫林煤时,发现海水向盆地的输入导致形成煤中高含量的硫及微量元素B,Mo和U,并终止了泥炭的堆积。在弥勒煤中,底部的煤分层中全硫的质量分数高于顶部和中部的煤分层,从底部至顶部硫的质量分数逐渐降低(图5),推测是海水逐渐退出煤盆地所致。在硫质量分数高于1%的煤样中,有机硫和硫酸盐硫分别占全硫的50%和40%,也表明了其具有海相成因的特点[9]。

图5 弥勒煤的灰分Aad(%),全硫St(%)、P2O5和CaO的质量分数(%),以及Ca/Mg,Sr/Ba,Th/U,V/Ni和Y/Ho质量分数比在剖面上的变化
Fig.5 Variation of ash yield Aad (%),concentrations of total sulfur St (%),P2O5 and CaO (%),as well as the ratios of Ca/Mg,Sr/Ba,Th/U,V/Ni and Y/Ho along the Mile coal seam depth
由于海水中的Mg2+质量分数远远高于Ca2+[56],Ca/Mg质量分数比可用作沉积环境的指示剂,反映沉积物水体的盐度。水体的盐度越高,Ca/Mg质量分数比越低[57-58]。本次研究的弥勒煤中Ca/Mg质量分数比为5.26(表1),与欧洲莱茵河附近受海水影响的褐煤中Ca/Mg质量分数比6[59]相当,也表明弥勒盆地的煤层在形成过程中受到了海水的影响。
沉积物中Sr和Ba的质量分数与水体的盐度有关[60-63]。全球现代海洋中Sr和Ba的平均质量浓度分别为8.0,0.013 mg/L,河流中平均质量浓度分别为0.07,0.02 mg/L[64]。因此Sr/Ba质量分数比可用来判别沉积环境的介质溶液盐度[65-66]。对于煤的沉积环境,该值可用以识别其受到淡水、海水或者两者的共同影响[25-26]。
弥勒样品(煤、夹矸、顶底板)中Sr/Ba质量分数比与Ad的相关性(图6)表明,低灰分(<40%,多数低于22%)样品中的Sr/Ba质量分数比>1,而高灰分样品(44%~80%)中Sr/Ba质量分数比<1,并且Sr/Ba质量分数比与灰分呈一定的负相关性。Sr/Ba质量分数比=1为煤层剖面上指示海水/淡水影响的临界值(图6)。因此,Sr/Ba质量分数比>1的含石膏的煤层(Ad<40%)受到了海水的影响,而Sr/Ba质量分数比<1的底板、顶板和夹矸(Ad>40%)形成主要受淡水的影响。由于海水中Sr的质量浓度比Ba的质量浓度高出100倍[61],因此,受淡水影响的沉积环境中,只要有少量海水的输入,就会导致该沉积环境介质溶液具有海水87Sr/86Sr特征[25-26]。此外,在煤层剖面上,Sr/Ba质量分数比和CaO的质量分数在下部煤层中较高,随后至煤层上部显著降低(图5),反映了海水输入影响减弱,而淡水输入影响增加的过程。

图6 弥勒煤中Sr/Ba质量分数比与煤的灰分(%)的关系(修改自SPIRO等[25])
Fig.6 Diagram of Sr/Ba values and ash yield (%) of the Mile coal seam (modified from SPIRO et al.[25])
煤中具有高质量分数P2O5的层位(位于1 394~1 546 cm和385~513 cm处)也具有高的CaO,Ca/Mg和Sr/Ba质量分数比(图5),因此高质量分数的P2O5与海水的强烈影响有关[67]。
Th/U比值可用作沉积环境的指示剂[68]。通常情况下,陆相风化产物中Th/U质量分数比较高(>7),海相黑色页岩和灰岩中该元素质量分数较低(<2)[69]。BOU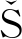 KA[70]指出,陆相沉积物中Th/U质量分数比>7,海相沉积物中Th/U质量分数比<7。因此,受海水输入影响较大煤中的Th/U质量分数比应低于未受海水影响或影响较小的煤层。在弥勒煤中,Th/U质量分数比为0.05~4.34,均值为1.43(表1)。从剖面底部至顶部,随着海水作用的逐渐减弱,Th/U质量分数比也呈现递增的趋势。在煤层深度1 300~1 400 cm处(图5),Th/U质量分数比骤然降低,与P2O5的质量分数以及Ca/Mg,Sr/Ba的质量分数比在该处出现的拐点一致,表明在该成煤阶段,海水对煤层的影响减弱的程度最为显著。同时,由于海水中相对富集可溶性氧化铀的化合物[68],因此在个别煤分层(ML-6,ML -11,ML -59)中U相对富集(最高为14 μg/g),也是泥炭层中海水输入的结果。
KA[70]指出,陆相沉积物中Th/U质量分数比>7,海相沉积物中Th/U质量分数比<7。因此,受海水输入影响较大煤中的Th/U质量分数比应低于未受海水影响或影响较小的煤层。在弥勒煤中,Th/U质量分数比为0.05~4.34,均值为1.43(表1)。从剖面底部至顶部,随着海水作用的逐渐减弱,Th/U质量分数比也呈现递增的趋势。在煤层深度1 300~1 400 cm处(图5),Th/U质量分数比骤然降低,与P2O5的质量分数以及Ca/Mg,Sr/Ba的质量分数比在该处出现的拐点一致,表明在该成煤阶段,海水对煤层的影响减弱的程度最为显著。同时,由于海水中相对富集可溶性氧化铀的化合物[68],因此在个别煤分层(ML-6,ML -11,ML -59)中U相对富集(最高为14 μg/g),也是泥炭层中海水输入的结果。
与Ni相比,V更倾向富集于贫氧的海水中[71-72],因此,海水中的V/Ni质量分数比高于淡水[73],并且,沉积岩中的V/Ni质量分数比可用于氧化-还原沉积环境的判别[26,71,74-80]。在弥勒煤层剖面上,V/Ni质量分数比从下部至上部逐渐降低,也指示了沉积环境由还原性海相环境向氧化性的淡水环境的转变。
由于水体中稀土元素Y和Ho的清除速率不同,特别是在海水中,Ho会被海洋颗粒物优先清除,导致海洋沉积物中Y/Ho质量分数比显著高于硅质碎屑沉积物[81-83]。因此,Y/Ho质量分数比可用于判断硅质碎屑沉积物(Y/Ho质量分数比25~30)和海相沉积物(Y/Ho质量分数比60~70)[26,81,83]。弥勒煤中Y/Ho质量分数比为18.67~64.00(表1),表明泥炭堆积过程中既有硅质碎屑的供给,又有海水的影响。Y/Ho质量分数比最大值位于煤层深度1 300~1 400 cm处(ML-34煤分层),随后比值显著降低(图5),指示陆源碎屑供给加强、海水作用的减弱,与本文中所讨论的其他参数异常特征一致。
在稀土元素方面,弥勒煤显示出La和Gd的正异常特征。La正异常是现代海水典型的稀土元素特征之一[84],弥勒煤中La的正异常可能指示了海水的影响。中国大部分煤通常具有较弱的Gd负异常特征[42]。煤中的Gd正异常主要受海水、热液和其他水体的影响[42]。本次研究的煤中尚未发现热液流体的存在以及受热液流体形成的矿物,因此该煤中Gd正异常可能是由于海水的输入所致。
HODELL等[85]测定了深海钻探项目(Deep Sea Drilling Project,DSDP)3个钻孔(519,588和607钻孔)261件保存完好的浮游有孔虫化石样品的87Sr/86Sr比值,并结合磁地层学、氧同位素地层学、生物地层学和稳定同位素地层学等手段精确测定的地层年代,绘制了24 Ma至今海水中87Sr/86Sr比值的变化曲线(图4(b))。弥勒煤中石膏的87Sr/86Sr比值与中新世早期海水中87Sr/86Sr变化一致,且呈递增的变化趋势,表明形成石膏的母质溶液来源于海水。煤中A,B和C组87Sr/86Sr比值的均值在HODELL等[85]绘制的曲线上对应的地质年代分别为18.6,20.5,21.7 Ma(图4(b)),表明研究区泥炭的堆积始于早中新世21.7 Ma,终止于18.6 Ma,整个沉积过程持续了约3 Ma。
弥勒盆地煤中的87Sr/86Sr比值对应的地质年龄(21.5 ~18.5 Ma)恰好处于特提斯海域的关闭和哀牢山—红河断裂带碰撞变形区形成的时间21 Ma[86]或22~17 Ma[87]时间段内。同时,同一地质历史时期内,研究区煤中的87Sr/86Sr比值与海水中87Sr/86Sr比值一致,表明本次研究的石膏中的锶同位素值并未受到其他因素的影响(如与盆地基底三叠系个旧组灰岩之间发生水-岩反应)。因此,该锶同位素比值能够反映出研究区气候和沉积环境的变化。
新生代以来,印度板块持续向北推移,欧亚板块则被动向南推挤。由于云南位于印度板块东侧,因此其仅显示向南的滑移推挤。印度板块北北东向的推挤,一方面使青藏地块东部向东和南东向扩展移动;另一方面,由于其西北端相对东北端向北运动缓慢,使印度板块本身发生逆时针方向旋转,产生向东的侧向挤压,形成哀牢山—红河左行走滑断裂带[88]。另外,印度板块东侧地块向南滑移的过程中,滇中地区表现出比两侧地块更大的滑移速度,导致沿东侧鲜水河、小江断裂带上发生明显的左行位移,形成当前的鲜水河—小江断裂系,并与哀牢山—红河断裂带交汇。本文研究区即位于该断裂系内[28]。与此同时,太平洋板块的俯冲方向由北北西转为北西西,促使原北东、北北东方向的断裂(如富源—弥勒、宣威—寻甸断裂带、弥勒—师宗断裂带),转为右旋剪切运动,并伴随着哀牢山—红河断裂带北部区域(包括本研究区弥勒盆地)的抬升。
弥勒盆地为山间内陆盆地,其与哀牢山—红河断裂带中的特提斯海域之间的水系连接可能为变形断裂带。泥炭堆积期,长期处于多阶段活动期的断裂带变形区,为海水输入至泥炭沼泽提供了通道。哀牢山—红河断裂带北部区域的抬升,使弥勒盆地煤层形成时受到海水的影响逐渐减弱。
(1)发现了在该陆相煤盆地泥炭堆积过程中海水入侵的新证据。石膏和自生黄铁矿的组成特征和赋存状态,均具有海相沉积特征。
(2)煤中指示沉积环境的敏感元素对Ca/Mg,Sr/Ba,Th/U,V/Ni,以及P2O5的质量分数,稀土元素La和Gd正异常,Y/Ho比值在煤层剖面上的变化特征,均表明海水的入侵,并且随着泥炭的堆积,海水的影响逐渐减弱,而淡水的影响和陆源碎屑的供给逐渐增强。
(3)在泥炭堆积期间,弥勒盆地处于多期活动阶段的断裂带变形区内,该盆地通过哀牢山—红河断裂带与特提斯海相连接,为海水输入至陆地泥炭堆积地提供了通道;同时,哀牢山—红河断裂带北部区域的抬升,使弥勒盆地泥炭堆积时受到海水的影响减小。
[1] WHITE D,THIESSEN R. The origin of coal[R]. Washington DC:U. S. Department of the Interior,Bureau of Mines,1913:Bulletin 38.
[2] CHOU Chen lin. Geochemistry of sulfur in coal[A]. ORR W L,WH-ITE C M. Geochemistry of Sulfur in Fossil Fuels[C]. Washington,DC:American Chemical Society,1990,429:30-52.
[3] DIESSEl C F K. Coal-bearing depositional systems[M]. Berlin,Heidelberg:Springer-Verla,1992.
[4] SPEARS D A,RIPPON J H,CAVENDER P F. Geological controls on the sulphur distribution in British Carboniferous coals:A review and reappraisal[J]. International Journal of Coal Geology,1999,40(1):59-81.
[5] DAI Shifeng,REN Deyi,TANG Yuegang,et al. Distribution,isotopic variation and origin of sulfur in coals in the Wuda coalfield,Inner Mongolia,China[J]. International Journal of Coal Geology,2002,51:237-250.
[6] KALKREUTH W,HOLZ M,KERN M,et al. Petrology and chemistry of Permian coals from the Paraná Basin:1. SantaTerezinha,Leão-Butiá and Candiota Coalfields,Rio Grande do Sul,Brazil[J]. International Journal of Coal Geology,2006,68:79-116.
[7] WILLIAMS E G,KEITH M L. Relationship between sulfur in coals and the occurrence of marine roof beds[J]. Economic Geology,1963,58:720-729.
[8] CHOU Chenlin. Geologic factors affecting the abundance,distribut-ion,and speciation of sulfur in coals[A]. Yang Q. Geology of Fossil Fuels—coal[C]. The Netherlands,Utrecht:Proceedings of the 30th International Geological Congress,Part B,1997,18:47-57.
[9] CHOU Chen lin. Sulfur in coals:A review of geochemistry and ori-gins[J]. International Journal of Coal Geology,2012,100:1-13.
[10] STANGER R,WALL T. Sulphur impacts during pulverised coal co-mbustion in oxy-fuel technology for carbon capture and storage[J]. Progress in Energy and Combustion Science,2011,37:69-88.
[11] FINKELMAN R B,PALMER C A,WANG Peipei. Quantification of the modes of occurrence of 42 elements in coal[J]. International Journal of Coal Geology,2018,185:138-160.
[12] DAI Shifeng,YANG Jianye,WARD C R,et al. Geochemical and mineralogical evidence for a coal-hosted uranium deposit in the Yili Basin,Xinjiang,northwestern China[J]. Ore Geology Review,2015,70:1-30.
[13] KANG Jian,LI Xiao. Modes of occurrence of minerals in the Carboniferous coals from the Wuda Coalfield,northern China:With an emphasis on apatite formation[J]. Arabian Journal of Geoscience,2016,9:606.
[14] DAI Shifeng,REN Deyi,ZHOU Yiping,et al. Mineralogy and geochemistry of a super high-organic-sulfur coal,Yanshan Coalfield,Yunnan,China:Evidence for a volcanic ash component and influence by submarine exhalation[J]. Chemical Geology,2008,255:182-194.
[15] LEI Jiajin,REN Deyi,TANG Yuegang,et al. Sulfur-accumulating model of super high organosulfur coal from Guiding,China[J]. Chinese Science Bulletin,1994,39 (21):1817-1821.
[16] SHAO Longyi,JONES T,GAYER R,et al. Petrology and geochemistry of the high-sulphur coals from the Upper Permian carbonate coal measures in the Heshan Coalfield,southern China[J]. International Journal of Coal Geology,2003,55:1-26.
[17] TURNER B R,RICHARDSON D. Geological controls on the sulphur content of coal seams in the Northumberl and Coalfield,northeast England[J]. International Journal of Coal Geology,2004,60:169-196.
[18] BERNER R A. The synthesis of framboidal pyrite[J]. Economic Geology,1969,64:383-384.
[19] SWEENEY R E,KAPLAN I R. Pyrite framboid formation:Laboratory synthesis and marine sediments[J]. Economic Geology,1973,68:618-634.
[20] HOU Xiaoqiang,REN Deyi,MAO Heling,et al. Application of imaging TOF-SIMS to the study of some coal macerals[J]. International Journal of Coal Geology,1995,27:23-32.
[21] ZENG Rongshu,ZHUANG Xinguo,KOUKOUZAS N,et al. Characterization of trace elements in sulphur-rich Late Permian coals in the Heshan coal field,Guangxi,South China[J]. International Journal of Coal Geology,2005,61:87-95.
[22] 韩德馨,任德贻. 中国煤岩学[M]. 徐州:中国矿业大学出版社,1996.
[23] GOODARZI F,SWAINE D J. Paleoenvironmental and environmen-tal implications of the boron content of coals[J]. Geological Survey of Canada Bulletin,1994,471:1-46.
[24] SPEARS D A,BORREGO A G,COX A,et al. Use of laser ablation ICP-MS to determine trace element distributions in coals,with special reference to V,Ge and Al[J]. International Journal of Coal Geology,2007,72:165-176.
[25] SPIRO Baruch,LIU Jingjing,DAI Shifeng,et al. Marine derived87Sr/86Sr in coal,a new key to geochronology and palaeoenvironment:Elucidation of the India-Eurasia and China-Indochina collisions in Yunnan,China[J]. International Journal of Coal Geology,2019,215:103304.
[26] DAI Shifeng,BECHTEL A,EBLE C F,et al. Recognition of peat depositional environments in coal:A review[J]. International Journal of Coal Geology,2020,219:103383.
[27] 王祖关. 云南岩相古地理图集[M]. 昆明:云南科技出版社,1995.
[28] SCHOENBOHM L M,BURCHFIEL B C,CHEN L Z,et al. Miocene to present activity along the Red River fault,China,in the context of continental extrusion,upper crustal rotation,and lower-crustal flow[J]. Geological Society of America Bulletin,2006,118:672-688.
[29] LIU Jingjing,WARD C R,GRAHAM I T,et al. Modes of occurrence of non-mineral inorganic elements in lignites from the Mile Basin,Yunnan Province,China[J]. Fuel,2018,222:146-155.
[30] LIU Jingjing,DAI Shifeng,HOWER J C,et al. Stable isotopes of organic carbon,palynology,and petrography of a thick low-rank Miocene coal within the Mile Basin,Yunnan Province,China:Implications for palaeoclimate and sedimentary conditions[J]. Organic Geochemistry,2020,149:104103.
[31] ASTM Standard D3174-11,Test method for ash in the analysis sample of coal and coke[S].
[32] ASTM Standard D3177-02 (Reapproved 2007),Test methods for total sulfur in the analysis sample of coal and coke[S].
[33] LI Chaofeng,GUO Jinghui,CHU Zhuyin,et al. Direct high-precision measurements of the 87Sr/86Sr isotope ratio in natural water without chemical separation using thermal ionization mass spectrometry equipped with 1012 Ω resistors[J]. Analytical Chemistry,2015,87:7426-7432.
[34] DAI Shifeng,WANG Xibo,SEREDIN V V,et al. Petrology,mineralogy,and geochemistry of the Ge-rich coal from the Wulantuga Ge ore deposit,Inner Mongolia,China:New data and genetic implications[J]. International Journal of Coal Geology,2012,90/91:72-99.
[35] RAO C P,GLUSKOTER H J. Occurrence and distribution of minerals in Illinois coals[J]. Illinois State Geological Survey,Circular,1973,476:56.
[36] WARD C R. Analysis and significance of mineral matter in coal seams[J]. International Journal of Coal Geology,2002,50:135-168.
[37] WARD C R. Analysis,origin and significance of mineral matter in coal:An updated review[J]. International Journal of Coal Geology,2016,165:1-27.
[38] DAI Shifeng,REN Deyi,CHOU Chen lin,et al. Geochemistry of trace elements in Chinese coals:A review of abundances,genetic types,impacts on human health,and industrial utilization[J]. International Journal of Coal Geology,2012,94:3-21.
[39] YUDOVICH Y,KETRIS M P. Estimations of Clarkes for carbonaceousbiolithes:World averages for trace element contents in black shales and coals[J]. International Journal of Coal Geology,2009,78:135-148.
[40] SEREDIN V V,DAI Shifeng. Coal deposits as potential alternative sources for lanthanides and yttrium[J]. International Journal of Coal Geology,2012,94:67-93.
[41] BAU M,KOSCHINSKY A,DULSKI P. Comparison of the partitioningbehaviours of yttrium,rare earth elements,and titaniumbetween hydrogeneticmarine ferromanganese crusts and seawater[J]. Geochimica et Cosmochimica Acta,1996,60:1709-1725.
[42] DAI Shifeng,GRAHAM I T,WARD C R. A review of anomalous rare earth elements and yttrium in coal[J]. International Journal of Coal Geology,2016,159:82-95.
[43] LEYBOURNE M I,GOODFELLOW W D,BOYLE D R,et al. Rapid development of negative Ce anomalies in surface waters and contrasting REE patterns in groundwaters associated with Zn-Pb massive sulfide deposits[J]. Applied Geochemistry,2000,15:695-723.
[44] ZHANG Xiaoyu,ZHANG Fuyuan,CHEN Xin,et al. REEs fractionation and sedimentary implication in surface sediments from eastern South China Sea[J]. Journal of Rare Earths,2012,30:621-626.
[45] RICKARD D. The origin of framboids[J]. Lithos,1970,3:269-293.
[46] RICKARD D T. Kinetics and mechanisms of pyrite formation at low temperatures[J]. America Journal of Science,1975,275:636-652.
[47] SMITH J W,BATTS B D. The distribution and isotopic composition of sulfur in coal[J]. Geochimica et Cosmochimica Acta,1974,38:121-133.
[48] BOCTOR N Z,KULLERUD G,SWEANY J L. Sulfide minerals in Seelyville Coal III,Chinook Mine,Indiana[J]. Mineral Deposita,1976,11:249-266.
[49] JAVOR B J,MOUNTJOY E W. Late Proterozoic microbiota of the Miette Group,southern British Columbia[J]. Geology,1976,4(2):111-119.
[50] PEARSON M J. Geochemistry of the Hepworth Carboniferous sediment sequence and origin of the diagenetic iron minerals and concretions[J]. Geochimica et Cosmochimica Acta,1979,43:927-941.
[51] CHOU Chen lin. Relationship between geochemistry of coal and the nature of strata overlying the Herrin coal in the Illinois Basin,U. S. A[J]. Memoir of the Geological Society of China,1984,6:269-280.
[52] WHATELEY M K G,TUNCALI E. Origin and distribution ofsulphur in the Neogene Beypazari lignite basin,central Anatolia,Turkey[A]. WHATELEY M K G,SPEARS D A. European Coal Geology[C]. UK:Geological Society Special Publication,1995,82:307-323.
[53] LUTHER III G W. Pyrite synthesis via polysulfide compounds[J].
Geochimica et Cosmochimica Acta,1991,55:2839-2849.
[54] CASAGRANDE D J. Sulphur in peat and coal[A]. Scott A C. Coal and Coal-Bearing Strata:Recent Advances[C]. London:Geological Society Special Publication,1987,32:87-105.
[55] CHOU Chenlin. Relationship between geochemistry of coal and the nature of strata overlying the Herrin Coal in the Illinois Basin,USA[J]. Memoir of the Geological Society of China,1984,6:269-280.
[56] MOZLEY P S. Relation between depositional environment and the elemental composition of early diagenetic siderite[J]. Geology,1989,17(8):704-706.
[57] PEARSE A S,GUNTER G. Salinity[J]. Geological Society of Ame-rica,Memoir,1957,67(1):129-158.
[58] FERGUSON J E,HENDERSON G M,KUCERA M,et al. Systematic change of foraminiferal Mg/Ca ratios across a strong salinity gradient[J]. Earth Planetary Science Letters,2008,265:153-166.
[59] 同济大学海洋地质系. 海、陆相地层辨认标志[M]. 北京:科学出版社,1980.
[60] YANG Hao,CHEN Zhongqiang,WANG Yongbiao,et al. Composition and structure of microbialite ecosystems following the end-Permian mass extinction in South China[J]. Palaeogeography,Palaeoclimatology,Palaeoecology,2011,308:111-128.
[61] 蓝先洪,马道修,徐明广,等. 珠江三角洲若干地球化学标志及指相意义[J]. 海洋地质与第四纪地质,1987(1):41-51.
LAN Xianhong,MA Daoxiu,XU Mingguang,et al. Some geochemical indicators of the Pearl River Delta and their facies significance[J]. Marine Geology & Quaternary Geology,1987 (1):41-51.
[62] CHEN Zhongyuan,CHEN,Zhenglou,ZHANG Weigou. Quaternary stratigraphy and trace-element indices of the Yangtze Delta,Eastern China,with special reference to marine transgressions[J]. Quaternary Research,1997,47:181-191.
[63] 王益友,郭文莹,张国栋. 几种地球化学标志在金湖凹陷阜宁群沉积环境中的应用[J]. 同济大学学报,1979(2):51-60.
WANG Yiyou,GUO Wenying,ZHANG Guodong. Application of some geochemical indicators in determining of sedimentary environment of the Funing Group (Paleogene),Jin-Hu Depression[J]. Journal of Tongji University,1979(2):51-60.
[64] REIMANN C,DE CARITAT P. Chemical elements in the environment:Factsheets for the Geochemist and Environmental Scientist[M]. Berlin Heidelberg:Springer-Verlag,1998.
[65] SCHMITZ B,ABERG G,WERDELIN L,et al.87Sr/86Sr,Na,F,Sr,and La in skeletal fish debris as a measure of the paleosalinity of fossil-fish habitats[J]. Geological Society of America Bulletin,1991,103:786-794.
[66] ARAI T,HIRATA T. Differences in the trace element deposition in otoliths between marine-and freshwater-resident Japanese eels,Anguilla japonica,as determined by laser ablation ICPMS[J]. Environmental Biology of Fishes,2006,75:173-182.
[67] MORRIS A W,BALE A W,HOWLAND R J M. Nutrient distribution in an Estuary:Evidence of chemical precipitation of dissolved silicate and phosphate[J]. Estuarine Coastal and Shelf Science,1981,12:205-216.
[68] VANder Flier E,FYFE W S. Uranium-thorium systematics of two Canadian coals[J]. International Journal of Coal Geology,1985,4:335-353.
[69] ADAMS J A S,WEAVER C E. Thorium to uranium ratios as indicators of sedimentary processes:an example of geochemical facies[J]. American Association of Petroleum Geologists,1958,42:387-430.
[70] BOU KA V. Geochemistry of coal[M]. Amsterdam:Elesvier,1981:284.
KA V. Geochemistry of coal[M]. Amsterdam:Elesvier,1981:284.
[71] LEWAN M D. Factors controlling the proportionality of vanadium and nickel ratios in crude oils[J]. Geochimica et Cosmochimica Acta,1984,48:2231-2238.
[72] PETERS K E,MOLDOWAN J M. The Biomarker Guide:Interpreting Molecular Fossils in Petroleum and Ancient Sediments[M]. New Jersey:Prentice-Hall,Inc. ,Englewood Cliffs,1993:363.
[73] 涂光炽. 地球化学[M]. 上海:上海科学技术出版社,1984.
[74] HATCH J R,LEVENTHAL J S. Relationship between inferred redox potential of the depositional environment and geochemistry of the Upper Pennsylvanian (Missourian) Stark Shale Member of the Dennis Limestone,Wabaunsee County,Kansas,U. S. A[J]. Chemical Geology,1992,99:65-82.
[75] JONES B,MANNING D A C. Comparison of geochemical indices used for the interpretation ofpalaeoredox conditions in ancient mudstones[J]. Chemical Geology,1994,111:111-129.
[76] RIMMER S M. Geochemicalpaleoredox indicators in Devonian-
Mississippian black shales,central Appalachian Basin (USA)[J]. Chemical Geology,2004,206:373-391.
[77] ADEGOKE A K,ABDULLAH W H,HAKIMI M H,et al. Trace elements geochemistry of kerogen in Upper Cretaceous sediments,Chad (Bornu) Basin,northeastern Nigeria:origin and paleo-redox conditions[J]. Journal of African Earth Sciences,2014,100:675-683.
[78] ZHAO Jianhua,JIN Zhijun,JIN Zhenkui,et al. Applying sedimentary geochemical proxies for paleoenvironment interpretation of organic-rich shale deposition in the Sichuan Basin,China[J]. International Journal of Coal Geology,2016,163:52-71.
[79] EBLE C F,GREB S F. Geochemical,petrographic and palynologic characteristics of two late middle Pennsylvanian (Asturian) coal-to-shale sequences in the eastern Interior Basin,USA[J]. International Journal of Coal Geology,2018,190:99-125.
[80] ZHANG Shaohua,LIU Chiyang,LIANG Hao,et al. Paleoenvironmental conditions,organic matter accumulation,and unconventional hydrocarbon potential for the Permian Lucaogou Formation organic-rich rocks in Santanghu Basin,NW China[J]. International Journal of Coal Geology,2018,185:44-60.
[81] WEBB G E,KAMBER B S. Rare earth elements in Holocene reefal microbialites:A new shallow seawater proxy[J]. Geochimica et Cosmochimica Acta,2000,64:1557-1565.
[82] MCLENNANS M,TAYLOR S R. Sedimentary rocks and crustal evolution:Tectonic setting and secular trends[J]. The Journal of Geology,1991,99:1-21.
[83] CHEN Jianbo,ALGEO T J,ZHAO Laishi,et al. Diagenetic uptake of rare earth elements by bioapatite,with an example from Lower Triassic conodonts of South China[J]. Earth-Science Reviews,2015,149:181-202.
[84] ELDERFIELD H,GREAVES M J. The rare earth elements in seawater[J]. Nature,1982,296:214-219.
[85] HODELL D A,MUELLER P A,GARRIDO J R. Variations in the strontium isotopic composition of seawater during the Neogene[J]. Geology,1991,19:24-27.
[86] SEARLE M. Role of the Red River Shear zone,Yunnan and Vietnam,in the continental extrusion of SE Asia[J]. Journal of Geological Society,2006,163:1025-1036.
[87] LELOUP P H,LACASSIN R,TAPPONNIER P,et al. The Ailao
Shan-Red River shear zone (Yunnan,China),Tertiary transform boundary of Indochina[J]. Tectonophysics,1995,251:3-84.
[88] CAI Jianxin,ZHANG Kaijun. A new model for the Indochina and
South China collision during the Late Permian to the Middle Triassic[J]. Tectonophysics,2009,467:35-43.