水煤气变换反应(WGS,CO(g)+H2O(g)→CO2(g)+H2(g))是CO去除和H2制备的途径[1],也是合成气转化过程中的重要反应,如甲醇合成[2]和费托合成[3]。常见的WGS反应催化剂有Au,Cu,Pd,Ni和Pt基等催化剂[4-8]。为了提高催化剂的活性,通常通过添加助剂[9-12]或改变载体[13-15]的方法。
过渡金属碳化物是一种金属间填充型化合物,是由碳原子填隙式融进过渡性金属的晶格中形成。由于其表面性质和催化活性类似于Pt等贵金属,目前广泛应用于催化加氢、脱氢、WGS和异构化等反应[16-17]。如,Au/α-MoC用于WGS反应时,反应速率和CO转化率分别是Cu/Zn/Al2O3催化剂的354倍和17.8倍[18],同时Au/α-MoC催化剂的活性明显优于Au/β-MoC。α-MoC对WGS反应起到了重要的作用,因此,笔者选取了α-MoC作为载体。
理论计算结果显示,α-MoC(111)载体对WGS反应没有催化效果,但是能够促进H2O的解离。当其负载Au和Cu后能够提高WGS反应在2种催化剂表面的活性[18-19]。Pd,Ni,Pt基催化剂也是WGS反应的常用催化剂,为了进一步了解α-MoC载体在WGS反应中的作用和WGS反应机理,笔者使用密度泛函理论(DFT)和动力学蒙特卡罗(KMC)方法系统的研究这3种金属负载α-MoC催化剂上的WGS反应过程,研究不同反应温度时的反应速率和催化转换频率(TOF),并对WGS反应催化剂的优化提供指导。
1 计算方法和模型
计算使用VASP软件[20-22],采用了GGA-PBE泛函求解电子交换相关能[23]。平面波截断能取值为 415 eV,布里渊区的k点选择 3×3×1[24]。在结构优化的过程中当力的变化小于0.1 eV/nm和能量的变化小于1.0×105 eV/atom时达到收敛标准。采用CI-NEB方法进行过渡态搜索,对达到收敛标准的过渡态进行频率分析,通过唯一虚频确定过渡态[25]。
首先对金属晶胞M(M=Ni,Pd,Pt)和α-MoC晶胞进行了优化,优化后对应的的晶格常数分别为aNi=0.351 3 nm,aPd=0.393 7 nm,aPt=0.396 7 nm,aα-MoC=0.437 0 nm,分别与实验值 aNi=0.352 4 nm,aPd=0.389 1 nm,aPt=0.392 4 nm和aMoC=0.427 8 nm接近[26-27]。因此,选择的计算方法和参数对本文的计算体系是合理的。α-MoC(111)采用7层3×3的平板模型,1.5 nm的真空层。由于H2O的快速解离,α-MoC表面的Mo位点被O占据[19],如图1所示,图1中top,hol,bri为吸附位点。计算过程中固定底面两层,其余原子弛豫。金属M4簇负载存在2种构型:平面簇构型(2d rhombic M4)和四面体簇(3d-tetrahedral M4),对应的吸附能分别为Ni4(2d:-8.41 eV;3d:-7.63 eV),Pd4(2d:-8.56 eV;3d:-6.77 eV),Pt4(2d:-10.20 eV;3d:-9.32 eV)。计算结果表明,平面簇构型更稳定,故仅考虑了2d-M4负载在α-MoC(111)表面。

图1 M4/α-MoC(111)的侧视图和俯视图
Fig.1 Top and side view of M4/α-MoC(111)
2 结果与讨论
对于水煤气变换反应,反应机理主要有2种:① 氧化还原机理:CO直接与H2O分解产生的氧结合,生成CO2;② 甲酸盐途径或者羧酸盐途径:CO与H2O分解产生的羟基结合,生成甲酸盐或羧酸盐中间体,最后分解成CO2[28-30]。反应过程中所涉及到的中间体,反应物和产物的最稳定吸附构型如图2所示。
图2和表1显示,反应物、中间体和产物在3类催化剂表面的最优吸附位相近,只有*H在Pt4/α-MoC(111)和*CO2在Ni4/α-MoC(111)的吸附例外。对中间体来说(*O,*OH,*CO2,*H2O,*CHOO,*COOH),它们在Pd4/α-MoC(111)和Pt4/α-MoC(111)的吸附能相近,吸附稳定性低于对应物种吸附在Ni4/α-MoC(111)。

图2 WGS反应所涉及的各中间体在Ni4/α-MoC(111)
的稳定吸附构型
Fig.2 Adsorption configurations of intermediates involved in the
WGS on the surfaces of Ni4/α-MoC(111)
表1 M4/α-MoC(111)面WGS反应过程中涉及到的中间体,反应物和产物的最优吸附位和吸附能
Table 1 Adsorption energy and adsorption site of the possible intermediates,reactants and productions
during the WGS reaction on M4/α-MoC(111)
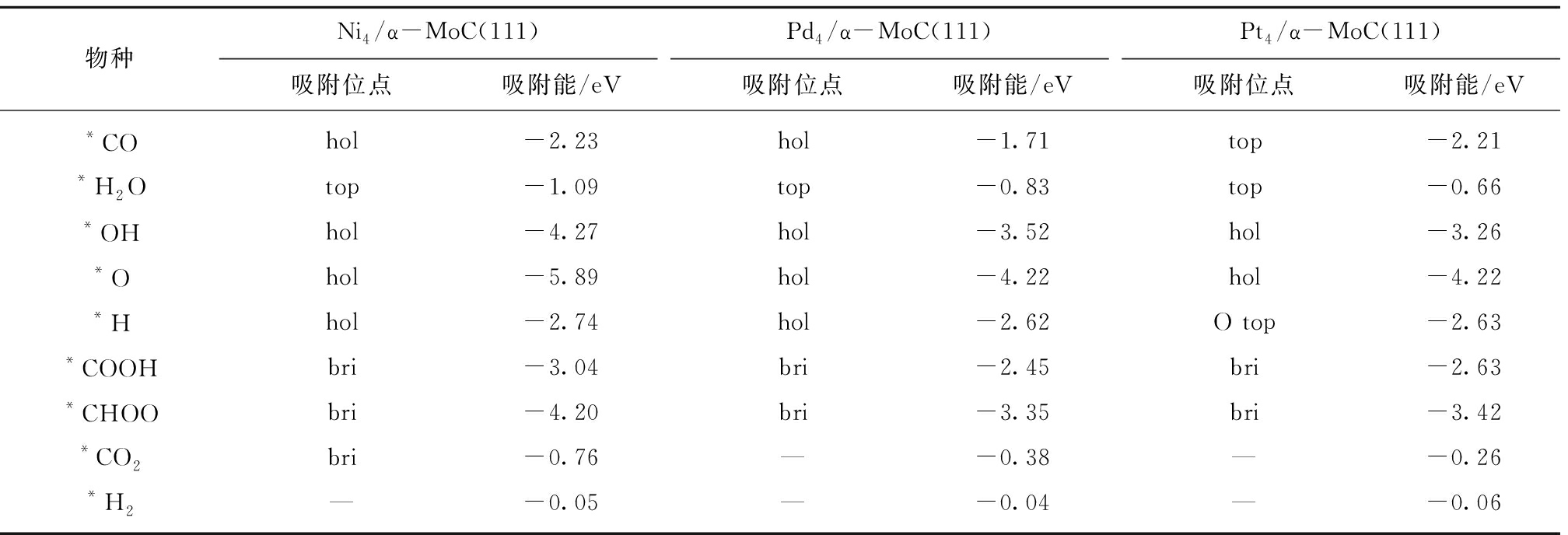
物种Ni4/α-MoC(111)吸附位点吸附能/eVPd4/α-MoC(111)吸附位点吸附能/eVPt4/α-MoC(111)吸附位点吸附能/eV*COhol-2.23hol-1.71top-2.21*H2Otop-1.09top-0.83top-0.66*OHhol-4.27hol-3.52hol-3.26*Ohol-5.89hol-4.22hol-4.22*Hhol-2.74hol-2.62O top-2.63*COOHbri-3.04bri-2.45bri-2.63*CHOObri-4.20bri-3.35bri-3.42*CO2bri-0.76—-0.38—-0.26*H2—-0.05—-0.04—-0.06
注:*为吸附物种。
反应物*CO吸附在Ni4/α-MoC(111)和Pd4/α-MoC(111)的吸附能相近(-2.23和-2.21 eV),而在Pt4/α-MoC(111)面的吸附稳定性低于Ni4/α-MoC(111)和Pd4/α-MoC(111)面。相比CO在Ni(111),Pd(111)和Pt(111)的吸附能,载体α-MoC提高了CO在Ni和Pd的吸附稳定性,但是降低了在Pt上的吸附稳定性[31-32]。对*H2O来说,载体α-MoC一定程度上提高了其在Ni,Pd和Pt表面的吸附稳定性[31-32]。
优化后,产物*CO2远离Pd4/α-MoC(111)和Pt4/α-MoC(111)面,但是其吸附在Ni4/α-MoC(111)的bri位,这是由于*CO2在Ni4/α-MoC(111)面的吸附稳定性强于Pd4/α-MoC(111)和Pt4/α-MoC(111)面。总体来说,相较Ni(111),Pd(111)和Pt(111),α-MoC(111)提高了CO2在3种催化剂表面的吸附稳定性[31-32]。对于H2来说,其在3个表面的吸附能力很弱,载体对其吸附基本没有影响。
*H2O的解离(*H2O+*→*OH+*H)是WGS反应的第1步,在Ni4/α-MoC(111)上*H2O解离的能垒为1.16 eV。对于氧化还原机理,*O生成存在2种方式:① *OH直接裂解(*OH+*→*O+*H)能垒Ea为0.95 eV;② *OH歧化反应(*OH+*OH→*H2O+*O)能垒为0.37 eV。最后,*CO氧化生成*CO2需克服1.62 eV的能垒。对于甲酸盐和羧酸盐路径,*CO与*OH反应,生成*CHOO(Ea=2.84 eV)或*COOH(Ea=1.61 eV)。结果表明,在Ni4/α-MoC(111)上氧化还原机理容易发生,速控步骤为*CO氧化。
在Pd4/α-MoC(111)上,*H2O克服1.23 eV能垒解离生成 *OH 和 *H。当发生氧化还原反应时,*OH歧化反应的能垒为0.35 eV,而*OH直接解离的能垒为1.02 eV。最后,*CO氧化生成*CO2的能垒为0.64 eV。在发生甲酸盐或羧酸盐路径中,*CO与*OH反应生成*CHOO和*COOH的能垒分别为1.64和1.28 eV。因此,在Pd4/α-MoC(111)上,WGS反应的氧化路径为主要路径,H2O的解离为速控步骤。
在Pt4/α-MoC(111)上,H2O的解离能垒为0.86 eV。当发生氧化还原反应时,*OH+*→*O+*H和*OH+*OH→*H2O+*O的反应能垒分别为1.28和0.35 eV,*CO氧化生成*CO2的能垒为0.81 eV。在发生甲酸盐或羧酸盐路径中,*CO与*OH反应生成*CHOO和*COOH的能垒分别为1.72和0.66 eV。由于生成*COOH的能垒远远低于生成*HCOO的能垒,因此*CO和*OH反应优先生成*COOH。最后,经*COOH与*OH反应生成*CO2和*H2O(Ea=0.42 eV)。
基于此,根据WGS反应的正逆反应能垒和不同温度时的指前因子,利用KMC研究了WGS反应在Ni4/α-MoC(111),Pd4/α-MoC(111)和Pt4/α-MoC(111)上的反应路径和催化转换频率。对于反应物和产物的吸附和脱附,考虑了熵的贡献[33]。KMC模型和计算参数等详细信息见文献[34]。
KMC结果显示,标准大气压(n(CO)∶n(H2O)=1∶1,物质的量之比)和反应温度为500 K时,在Ni4/α-MoC(111)和Pd4/α-MoC(111)催化剂上,水煤气变换的反应机理为氧化还原机理如图3所示,图3中,TS为过渡态。即CO(g)+H2O(g)→*CO+*H2O→*CO+*OH+*H→*CO+*O+*2H→*CO2+*H2→CO2(g)+H2(g)。需要指出的是,尽管*OH+*OH→*H2O+*O的反应能垒远远小于*OH+*→*O+*H的反应能垒,然而由于高的*H2O→*OH+*H反应能垒导致表面*OH覆盖度过低。因此,KMC结果显示*OH的直接解离是*O产生的反应途径。在Pt4/α-MoC(111)催化剂上,水煤气变换的反应路径为羧酸盐路径如图4所示,即CO(g)+2H2O(g)→*CO+2*H2O→*CO+2*OH+2*H→*COOH+*OH+2*H→*CO2 +*H2O+*H2 →CO2(g)+H2O(g)+H2(g)。由于H2和CO2的TOF相等,因此图5仅列出了H2的TOF(反应条件为一个大气压下,CO与H2O物质的量之比为1∶1)。总体来说,H2的TOF随反应温度的升高而升高。H2在Ni4/α-MoC(111)和Pd4/α-MoC(111)的TOF偏低。在500 K时催化剂单位活性位点上转换次数约为0和0.056 s-1,这是由于WGS反应过程中反应能垒较高造成的。对于Pt4/α-MoC(111),值得注意的是,400 K时,H2在催化剂单位活性位点上转换次数约为0,这是因为Pt4/α-MoC(111)上CO的脱附能较高(1.07 eV),活性位点被CO所覆盖。随着反应温度的升高,CO的脱附能(500 K下脱附能为0.83 eV)降低,H2在催化剂单位活性位点上的TOF约为6.3 s-1,WGS反应发生。结果表明,*CO的强稳定性不利于WGS反应的进行。当反应温度为500 K时,H2的TOF的排序是:Pt4/α-MoC(111)≫Pd4/α-MoC(111)>Ni4/α-MoC(111)。因此,当3种金属负载在α-MoC载体上时,Pt4/α-MoC(111)催化剂活性最好。同时,其反应活性高于Pt/Al2O3(TOF≈0.7 s-1)和Pt/TiO2(TOF≈0.2 s-1)[35],这表明Pt/α-MoC是WGS反应的高活性催化剂。

图3 WGS反应在Ni4/α-MoC(111),Pd4/α-MoC(111)
上反应势能
Fig.3 Potential energy of WGS on Ni4/α-MoC(111) and
Pd4/α-MoC(111)
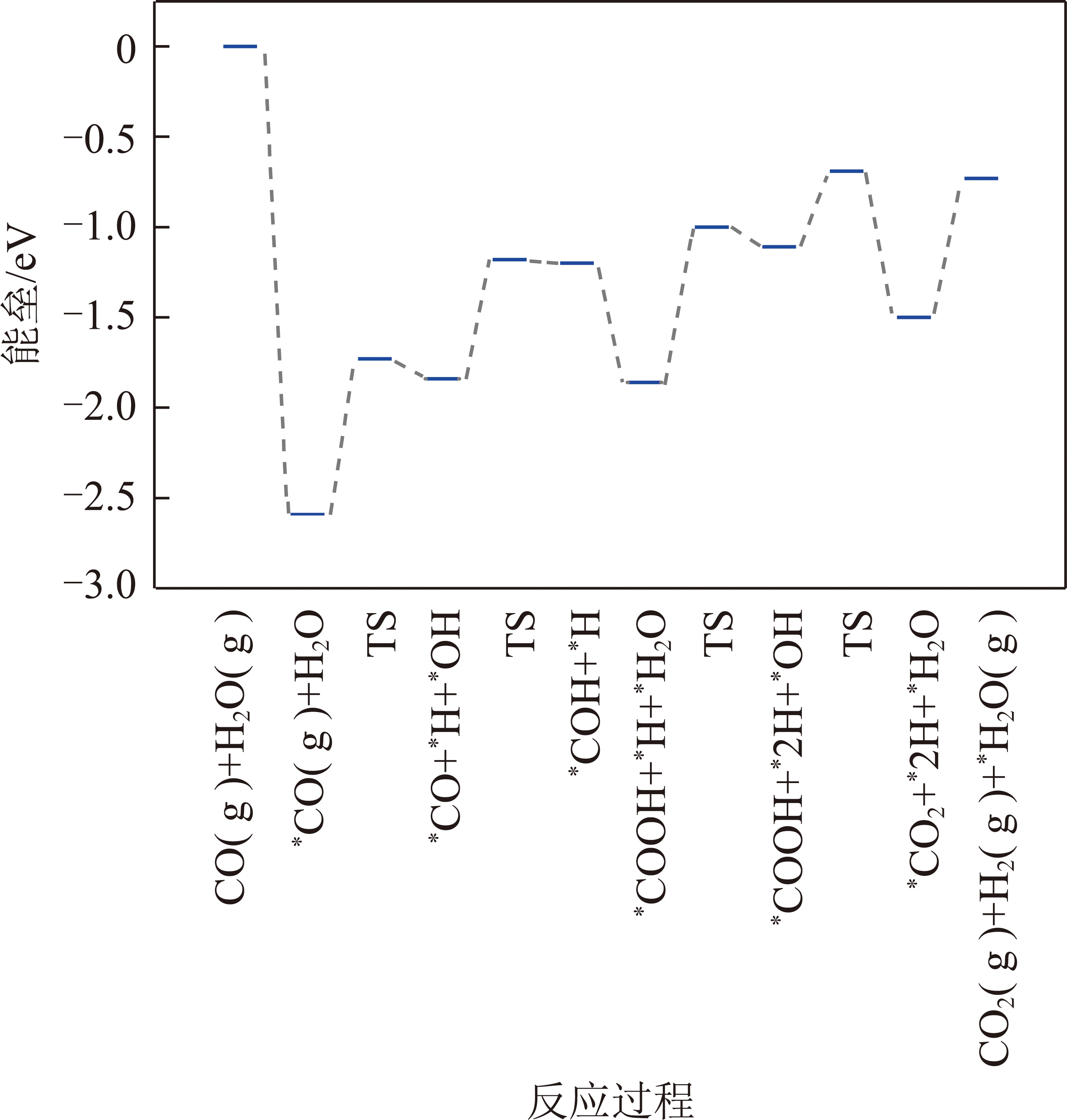
图4 WGS反应在Pt4/α-MoC(111)上反应势能
Fig.4 Potential energy of WGS on Pt4/α-MoC(111)

图5 WGS反应在M4/α-MoC(111)上的H2的TOF
Fig.5 H2 TOF for WGS reaction on M4/α-MoC(111)
3 结 论
(1)通过密度泛函理论和动力学蒙特卡洛方法探究了过渡金属Ni,Pd,Pt负载α-MoC催化剂的WGS反应的反应机理和活性。
(2)DFT计算结果发现,在Ni4/α-MoC(111)和Pd4/α-MoC(111)上,WGS反应的反应路径为氧化还原路径为CO(g)+H2O(g)→*CO+*H2O→*CO+*OH+*H→*CO+*O+*2H→*CO2+*H2→CO2(g)+H2(g);在Pt4/α-MoC(111)催化剂上,WGS反应通过羧酸盐路径发生,即CO(g)+2H2O(g)→*CO+2*H2O→*CO+2*OH+2*H→*COOH+*OH+2*H→*CO2+*H2O+*H2→CO2(g)+H2O(g)+H2(g)。
(3)动力学模拟结果表明,Pt4/α-MoC(111)具有较高的催化活性。标准大气压下,反应温度在400~500 K,H2O与CO的物质的量之比为1时,H2的TOF排序:Pt4/α-MoC(111)≫Pd4/α-MoC(111)>Ni4/α-MoC(111),Pt/α-MoC是WGS反应的高活性催化剂。
[1] ZHU M,WACHS I E.Iron-based catalysts for the high-temperature water-gas shift (HT-WGS) Reaction:A Review[J].ACS Catalysis,2016,6(2):722-732.
[2] LIU Y M,LIU J T,LIU S H,et al.Reaction mechanisms of methanol synthesis from CO/CO2 hydrogenation on Cu2O(111):Comparison with Cu(111)[J].Journal of CO2 Utilization,2017,20:59-65.
[3] KOBAYASHI K,ATSUMI R,MANAKA Y,et al.Effect of the TiO2 crystal structure on the activity of TiO2-supported platinum catalysts for ammonia synthesis via the NO-CO-H2O reaction[J].Catalysis Science Technology,2019,9(11):2898-2905.
[4] CLAY J P,GREELEY J P,RIBEIRO F H,et al.DFT comparison of intrinsic WGS kinetics over Pd and Pt[J].Journal of Catalysis,2014,320:106-117.
[5] YAO S Y,XU W Q,JOHNSTON-PECK A C,et al.Morphological effects of the nanostructured ceria support on the activity and stability of CuO/CeO2 catalysts for the water-gas shift reaction[J].Physical Chemistry Chemical Physics,2014,16(32):17183-17195.
[6] 任凯,楚金.钴钼催化剂在水煤气变换中的应用[J].化工设计通讯,2018,44(4):7-23.
REN Kai,CHU Jin.Application of cobalt molybdenum catalyst in water gas shift[J].Chemical Engineering Design Communications,2018,44(4):7-23.
[7] ARANIFARD S,AMMAL S C,HEYDEN A.On the importance of the associative carboxyl mechanism for the water-gas shift reaction at Pt/CeO2 interface sites[J].The Journal of Physical Chemistry C,2014,118(12):6314-6323.
[8] MA W P,JACOBS G,KEOGH R A,et al.Fischer-Tropsch synthesis:Effect of Pd,Pt,Re,and Ru noble metal promoters on the activity and selectivity of a 25%Co/Al2O3 catalyst[J].Applied Catalysis,A:General,2012,437/438:1-9.
[9] 朱龙雏,王亦飞,张志丰,等.煤灰和煤焦对气化合成气水煤气变换反应特性的影响[J].煤炭学报,2020,45(9):3293-3300.
ZHU Longchu,WANG Yifei,ZHANG Zhifeng,et al.Effect of coal ash and coal char on gasification syngas water gas shift rection[J].Journal of China Coal Society,2020,45(9):3293-3300.
[10] 史立杰,孙玉琢,李晨佳,等.变换催化剂的密度泛函理论研究进展[J].工业催化,2019,27(5):11-15.
SHI Lijie,SUN Yuzhuo,LI Jiachen,et al.Progress indensity functional theory study of shift catalysts[J].Industrial Catalysis,2019,27(5):11-15.
[11] 张中怀,陈韩,公丹丹,等.K掺杂对Co-CeO2催化剂逆水煤气变换反应性能的影响[J].广州化工,2020,48(14):50-52.
ZHANG Zhonghuai,CHEN Han,GONG Dandan,et al.Influence of K doping on the reaction performance of Co-CeO2 catalyst in reverse water-gas shift reaction[J].Guangzhou Chemical Industry,2020,48(14):50-52.
[12] 苑慧敏,王天成,张永军,等.Fe2O3对CuO/CeO2水煤气变换催化剂的改性研究[J].天然气化工,2019,44(1):28-29,35.
YUAN Huimin,WANG Tiancheg,ZHANG Yongjun,et al.Effect of precipitation temperature on activity and thermal stability of CuO/CeO2 catalyst for water gas shift reaction[J].Natural Gas Chemical Industry,2019,44(1):28-29,35.
[13] 张燕杰,陈崇启,詹瑛瑛,等.Y修饰CuO/ZrO2催化剂高效催化水煤气变换反应制氢[J].燃料化学学报,2017,45(9):1137-1145.
ZHANG Yanjie,CHEN Chongqi,ZHAN Yingying,et al.Highly active Y-promoted CuO/ZrO2 catalysts for the production of hydrogen through water-gas shift reaction[J].Journal of Fuel Chemistry and Technology,2017,45(9):1137-1145.
[14] 范言语,马骏驰,殷玲,等.ZrO2修饰的 CuO/Fe2O3水煤气变换催化剂的性能测试与表征[J].工业催化,2016,24(1):34-40.
FAN Yanyu,MA Junchi,YIN Ling,et al.Catalytic performance and characterization of ZrO2-modified CuO/Fe2O3 catalysts in water-gas shift reaction[J].Inoustrial Catalysis,2016,24(1):34-40.
[15] SABNIS K D,CUI Y R,AKATAY M C,et al.Water-gas shift catalysis over transition metals supported on molybdenum carbide[J].Journal of Catalysis,2015,331:162-171.
[16] HENRY H,WU H,CHEN J G.Surface chemistry of transition metal carbides[J].Chemical Reviews,2005,105(1):185-212.
[17] RODRIGUEZ J A,ILLAS F.Activation of noble metals on metal-carbide surfaces:Novel catalysts for CO oxidation,desulfurization and hydrogenation reactions[J].Physical Chemistry Chemical Physics,2012,14(2):427-438.
[18] YAO S,ZHANG X,ZHOU W,et al.Atomic-layered Au clusters on Au-MoC as catalysts for the low-temperature water-gas shift reaction[J].Science,2017,357(6349):389-393.
[19] ZOU X Y,MI L,ZUO Z J,et al.DFT study the water-gas shift reaction over Cu/α-MoC surface[J].Journal of Molecular Modeling,2020,26(9):237.
[20] KRESSE G,FURTHMULLER J.Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set[J].Physical Review B,1996,54,11169-11186.
[21] BLOCHL P E.Projector augmented-wave method[J].Physical Review B,1994,50(24):17953-17979.
[22] KRESSEG,HAFNER J.Ab initio molecular dynamics for liquid metals[J].Physical Review B,1993,47:558-561.
[23] PERDEW J P,BURKE K,ERNZERHOF M.Generalized gradient approximation made simple[J].Physical Review Letters,1996,77(18):3865-3868.
[24] POSAD-APEREZ S,VINES F,RODRIGUEZ J A,et al.Structure and electronic properties of Cu nanoclusters supported on Mo2C(001) and MoC(001) surfaces[J].The Journal of Chemical Physics,2015,143:114704.
[25] HENKELMAN G,UBERUAGA B P,JONSSON H.A climbing image nudged elastic band method for finding saddle points and minimum energy paths[J].The Journal of Chemical Physics,2000,113(22):9901-9904.
[26] MILMAN V,WINKLER B,WHITE J A,et al.Electronic structure,properties,and phase stability of inorganic crystals:A pseudopotential plane-wave study[J].International Journal of Quantum Chemistry,2000,77(5):895-910.
[27] GUILLERMET A F,HAGLUND J,GRIMVALL G.Cohesive properties and electronic structure of 5d-transition-metal carbides and nitrides in the NaCl structure[J].Physical Review B,Condensed Matter,1993,48(16):11673-11684.
[28] 陈磊,倪刚,韩波,等.Fe3O4(111)面上的水煤气变换反应机理[J].化学学报,2011,69(4):393-398.
CHEN Lei,NI Gang,HAN Bo,et al.Mechanism of water gas shift reaction on Fe3O4(111) surface[J].Acta Chimica Sinica,2011,69(4):393-398.
[29] 毛江洪,倪哲明,潘国祥,等.Cu催化水煤气的变换反应机理[J].物理化学学报,2008,24(11):2059-2064.
MAO Jianghong,NI Zheming,PAN Guoxiang,et al.Mechanism of the copper-catalyzed water gas shift reaction[J].Acta Physico-chimica Sinica,2008,24(11):2059-2064.
[30] 梁湦,何秋月,孙宝珍.Cu2O(111)催化水煤气变换反应机理的理论研究[J].分子催化,2017,31(6):553-566.
LIANG Sheng,HE Qiuyue,SUN Baozhen.Reaction mechanisms of WGSR Catalyzed by Cu2O(111):A theoretical study[J].Journal of Molecular Catalysis,2017,31(6):553-566.
[31] POUR A N,TAYYARI S F.Water-gas-shift reaction over nickel catalysts:DFT studies and kinetic modeling[J].Structural Chemistry,2019,30(5):1843-1852.
[32] CLAY J P,GREELEY J P,RIBEIRO F H,et al.DFT comparison of intrinsic WGS kinetics over Pd and Pt[J].Journal of Catalysis,2014,320:106-117.
[33] SHEN V K,SIDERIUS D W,KREKELBERG W P,et al.NIST standard reference simulation website,NIST standard reference database number 173.National institute of standards and technology,Gaithersburg,MD[EB/OL].(2017-09-01)[2021-04-07].https://webbook.nist.gov/chemistry/.
[34] ZUO Z,LIU S,WANG Z,et al.Dry Reforming of Methane on Single-Site Ni/MgO Catalysts:Importance of Site Confinement[J].ACS Catalysis,2018,8(10):9821-9835.
[35] PAZMINO J H,SHEKHAR M,WILLIAMS W D,et al.Metallic Pt as active sites for the water-gas shift reaction on alkali-promoted supported catalysts[J].Journal of Catalysis,2012,286:279-286.
