近年来,北美页岩气开发的巨大成功吸引了全世界的注意。根据美国能源署(EIA)的报告,2019年美国页岩气产量达到7 158.4亿m3,占其全国天然气总产量的75%,到2050年该比例将达到90%[1]。我国页岩气储量达31.58万亿m3,位居世界第1位[2]。我国从2012年开始页岩气商业化开采以来产量逐年增加,到2020年产量达到200.4亿m3[3],其中大部分产自四川盆地。页岩气具有自生、自储的特点,其主要成分是甲烷。甲烷在储层温度和压力条件下为超临界流体。主要以吸附态、游离态和溶解态赋存在页岩储层中,其中吸附态甲烷可达50%~60%[4]。因此,客观、精确地评价超临界条件下甲烷吸附能力对于计算页岩气地质储量和制定开发方案具有重要意义。
高压等温吸附实验是评价页岩气吸附能力的主要手段。目前,国内外学者针对页岩气(甲烷)高压等温吸附已开展了广泛的实验研究。GASPARIK等[5-6]、SHABANI等[7]、周尚文等[8]、YANG等[9]、XIONG等[10]、TANG等[11-12]采用改进型的Langmuir模型分析了甲烷在页岩上的高压吸附;REXER等[13]、TOPOR等[14]、TIAN等[15]、PAN等[16]、LI等[17]采用改进型的Dubinin-Radushkevich(D-R)模型来表征甲烷在页岩上的超临界吸附。董银涛等[18]、盛茂等[19]、SONG等[20]采用2种吸附模型联合的方法表明超临界甲烷吸附既包括单层吸附,也包含微孔充填。上述模型要么将吸附相密度假设为定值(如常压沸点液体甲烷密度423 kg/m3 ,Van der Waals密度373 kg/m3),或将吸附相密度设为待拟合参数,这与实际情况明显不符[21]。此外,Langmuir模型,D-R模型均是以亚临界条件建立的,难以推广到超临界吸附。
ZHOU等[22-23]将等温吸附数据线性化处理后认为超临界气体只能发生单层吸附,分子模拟[10,21,24-25]却表明超临界甲烷吸附可能存在多层吸附,且第1层的密度高于其他层。常见的多层吸附模型如Bruauer-Emmet-Teller(BET)模型,Harkins-Jura(H-J)模型,Frenkel-Halsey-Hill(FHH)模型均存在固有缺陷。比如BET模型在Gibbs积分中存在偏差[26],H-J模型,FHH模型和D-R模型在低压力时均不能回归到亨利定律方程。上述模型及其改进形式均涉及到饱和蒸汽压力,在超临界条件下气体无法液化,不存在饱和蒸汽压力[27]。因此,超临界甲烷吸附机理还有待进一步研究。
Ono-Kondo格子模型起源于格子理论,与上述模型相比,其拥有以下几个方面的优点:① 可以预测所有已知的各种吸附曲线[28];② 没有对吸附相性质作任何假设,既适用于单层吸附也适用于多层吸附;③ 该模型可由严格的统计学或热力学方法推导得出,各参数物理意义明确;④ 同时适用于亚临界吸附和超临界吸附。与其他格子模型(如格子玻尔兹曼模型[29]相比,Ono-Kondo模型求解过程更为简捷。BI等[30]、周尚文等[31]、 ZHOU等[32]采用忽略了气体分子间相互作用的单层Ono-Kondo格子模型来拟合超临界甲烷在页岩上的吸附数据,并取得了较好的拟合效果。笔者通过开展高温高压重量法甲烷吸附实验,在不对吸附相性质作任何假设的情况下,利用Ono-Kondo格子模型对等温吸附曲线进行拟合,分析超临界甲烷吸附层数,吸附相密度,绝对吸附量以及吸附相体积,进一步阐明超临界页岩气吸附机理。
1 Ono-Kondo格子吸附模型
Ono-Kondo格子吸附模型将吸附层均匀地划分为若干个格子(图1),每个格子最多能吸附1个分子,若某个吸附位没有吸附分子,则被称为空穴。相邻吸附分子间相互作用能为E,吸附分子与页岩表面作用能为Es,根据经典热力学理论及平均场[33]近似可以得到
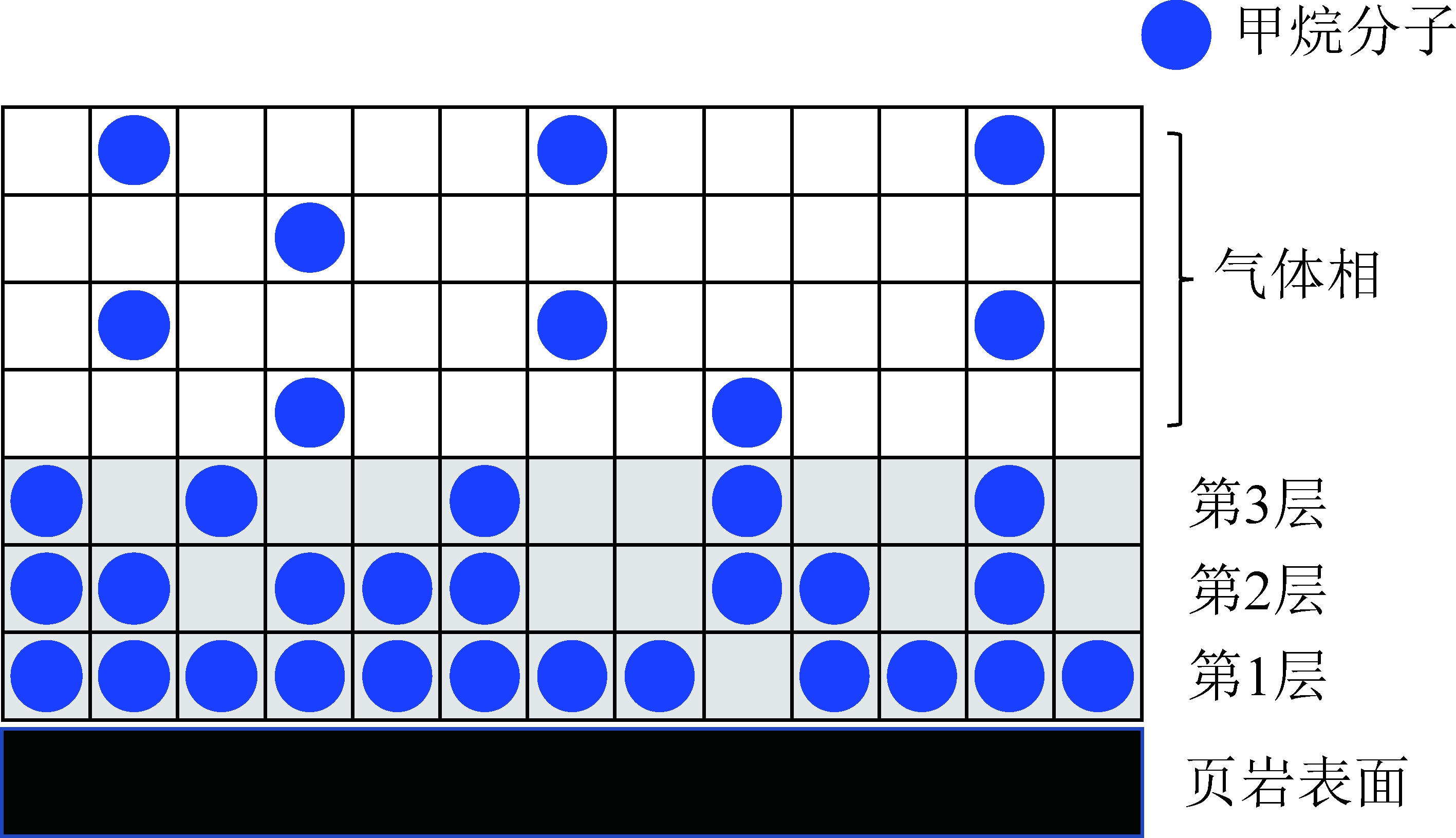
图1 Ono-Kondo格子吸附模型示意
Fig.1 Schematic diagram of the Ono-Kondo lattice model
![]()
(1)
![]()
(2)
![]()
(3)
![]()
(4)
其中,Xi为第i层被占据的吸附位比例;Xb为气体相分子占据的吸附位比例;z0为体积配位数;k为玻尔兹曼常数,1.38×10-23 J/K;T为绝对温度,K;z2为吸附层内配位数,z2=(z0-z1)/2,对于正六方体格子z0=8,z1=6,z2=1;z1为单吸附层配位数;ρi为第i层的吸附相密度;ρm为最大吸附相密度,即格子被全部占据时吸附相密度;ρb为气体相甲烷密度。过剩吸附量可以表示为
![]()
(5)
其中,C为页岩表面吸附位的总数,由页岩的化学成分和表面结构决定[34]。若只考虑单层吸附(i=1),并忽略吸附分子间相互作用(E=0),式(2)可写为Langmuir形式:

(6)
在低压力时,式(2)可以回归为亨利定律表达式
![]()
(7)
2 超临界甲烷等温吸附实验
选取四川盆地龙马溪组页岩粉碎成60~80目进行超临界甲烷吸附实验。实验仪器为德国Rubotherm重量法吸附仪(型号ISOSORP-HP),其核心部件为高精度的磁悬浮天平,质量测试精度可达10 μg,其最高测试压力为35 MPa±1 kPa,最高测试温度为(150±0.2) ℃。该装置通过分别称量真空和实验条件下3个不同质量(图2),可以同时测出气体相密度和过剩吸附量:① 在0点位置仅永磁体被悬挂,此时为称皮重同时校准微量天平;② 升起永磁体至测量点1,此时质量为页岩样品和样品篮之和;③ 继续升起永磁体至测量点2,此时测量页岩样品、样品篮、钛块(已知钛块体积为Vsk)的总质量。根据阿基米德原理,可以计算出气体相密度ρb[35]为

图2 重量法吸附仪不同称量位置示意
Fig.2 Schematic diagram of the gravimetric adsorption instru- ment at different measuring positions
![]()
(8)
其中,Msk,0,Msk分别为真空和实验条件下钛块的质量;MP1,0,MP1(ρ,T)分别为真空和实验条件下测量点1对应的质量;MP2,0,MP2(ρ,T)分别为真空和实验条件下测量点2对应的质量。与常规的测压法实验装置相比,重量法可以有效避免测压法中的累计误差和选择气体状态方程带来的误差[36]。实验测得的过剩吸附量nex可以表示为
![]()
(9)
其中,M为甲烷摩尔质量;V0为页岩样品和样品篮总体积;ms为页岩样品质量。根据Gibbs过剩吸附量的定义,nex还可以表示为
![]()
(10)
其中,nabs为绝对吸附量;Va为吸附相体积;ρa为吸附相密度。本文实验温度为40,60,80,100 ℃,最高实验压力为30 MPa。
3 结果与分析
3.1 实验测试结果
在浮力校正和计算过剩吸附量(式(9))时需要用到气体相甲烷密度,因此准确测定不同温度和压力下的气体相甲烷密度是准确测定过剩吸附量的先决条件。图3为重力法高压吸附仪所测得气体相密度与美国国家标准技术研究所(NIST)[37]提供的理论值对比。从图3可以看出,甲烷密度在实验温度下随压力呈现近似线性增加,重力法高压吸附仪可以精确测定不同压力和温度条件下的气体相密度,其平均绝对偏差分别为0.22%,0.18%,1.62%和0.16%。

图3 试验测得气体相甲烷密度和美国国家标准技术研究 所(NIST)结果对比
Fig.3 Results of bulk methane density.Dotes signify the determined data and the curves represent the data from NIST
超临界甲烷等温吸附实验结果如图4所示,过剩吸附量均先增加到一个极大值,然后过剩吸附量随压力升高而减小,该实验现象与其他学者所测得的页岩中甲烷超临界吸附一致[16-17,38-39]。其原因在于随着压力增加,式(10)中Va与ρb的乘积增加幅度超过nabs,从而导致过剩吸附量出现极值。当吸附相密度ρa与气体相密度ρb相等时,过剩吸附为0。最大过剩吸附量对应的压力随着温度升高而递增。40 ℃时最大过剩吸附量对应的压力约为12.5 MPa;当温度升高到100 ℃时,最大过剩吸附量对应的压力约为16 MPa。过剩吸附量均随着温度升高而降低,其主要原因是页岩气吸附为放热过程,温度升高不利于甲烷吸附[40]。

图4 超临界甲烷等温吸附实验结果及单层Ono-Kondo 格子模型拟合结果
Fig.4 Experimental data of supercritical methane adsorption and the fitting results of monolayer Ono-Kondo lattice model
3.2 吸附层数确定
结合分子模拟结果[41],分别分析单层和3层吸附的情况。图5展示了不同温度下3层Ono-Kondo格子模型计算得出的吸附相密度和实验测得的气体相甲烷密度。从图5可以看出,第1层吸附相密度大于气体相甲烷密度,第2层和第3层吸附相密度和气体相密度相当。其中第2层密度略低于第3层密度,该结果与文献中观察到的结果一致[42]。通过对图5中不同温度下吸附相密度的分析,可以得出第2层和第3层吸附相实际为气体相,其中第2层密度和气体相密度的细微差别可以归结为计算误差,即超临界甲烷吸附是单层吸附。

图5 不同温度下3层Ono-Kondo模型计算的吸附相密度和实验测得的气体相密度
Fig.5 Density of adsorbed CH4 evaluated from three-layers Ono-Kondo model and the determined density of bulk CH4 under different temperatures
表1列出了单层Ono-Kondo格子模型和3层Ono-Kondo格子模型的计算参数以及对实验数据的拟合结果。从表1中可以看出3层模型和单层模型的拟合效果相当,其中相关系数R2不小于0.999 6,均方根误差≤0.000 49 mmol/g,平均绝对偏差≤0.88%。该结果进一步表明超临界甲烷在页岩上的吸附是单层吸附。虽然分子模拟表明甲烷分子在中孔(2~50 nm)中可能形成多层吸附,但是分子模拟只针对单一孔径分布进行计算,并未考虑页岩真实孔径分布情况。页岩中既存在大量直径小于2 nm的微孔,也存在中孔和部分大孔[43-44]。由于势能的叠加作用,微孔孔壁附近强烈的气-固相互作用势能使得甲烷分子优先在微孔孔壁上吸附并形成吸附层。QI等[45]采用简化局部密度(SLD)模型分析了不同温度和压力下,甲烷在不同页岩孔隙(0.4~5.0 nm)中的密度分布曲线。该结果表明:在甲烷在页岩孔隙内只能形成单一吸附层,且该吸附层的位置不随温度和压力变化。
表1 不同温度下Ono-Kondo格子模型拟合结果
Table 1 Fitting results of the Ono-Kondo lattice model at various temperatures

温度/℃3层Ono-Kondo模型R2均方根误差/(mmol·g-1)平均绝对误差/%单层Ono-Kondo模型R2均方根误差/(mmol·g-1)平均绝对误差/%400.999 60.000 490.810.999 60.000 420.77600.999 80.000 320.700.999 70.000 430.88800.999 90.000 240.620.999 80.000 290.651000.999 80.000 270.670.999 80.000 270.75
与忽略气体分子相互作用的Langmuir模型[9,17,46],简化的Ono-Kondo格子模型[42,47]相比,本文的实验压力和拟合精度都更高,主要是本文所采用的重量法吸附仪比体积法精度更高;此外,本文将最大吸附相密度ρm和相邻吸附气体分子间相互作用势能E作为待拟合参数,能更加真实反应吸附剂和吸附质之间的相互作用。
3.3 温度对吸附的影响
从图6可以看出,吸附分子间排斥能E为正值,页岩与甲烷分子间吸附能Es为负值,且E/k和Es/k的绝对值、吸附位总数C和最大吸附相密度ρm均随着实验温度升高而降低。由于页岩表面的非均质特性,温度升高使得部分弱吸附点位可能会失去已经吸附的气体分子,从而导致吸附能力下降[48];另一方面,升高温度会使甲烷分子热运动更剧烈,动能增加,使得甲烷分子逃脱页岩壁的束缚力概率增大[49]。因此,模型中C值随温度升高而降低。

图6 模型参数随实验温度变化
Fig.6 Variation of model parameters versus experimental temperatures
在压力相同的条件下,温度升高导致页岩、吸附相、气体相产生宏观上的热膨胀。从微观角度上看,温度升高导致分子与分子间距离增大。结合12-6 Lennard-Jones势能曲线(图7)可以看出:随着温度升高,分子核间距增大,导致甲烷分子间的排斥作用能E减弱。同时,页岩和甲烷分子间的吸附作用能(Es的绝对值)也减弱。因此,模型中E/k和Es/k绝对值均随温度升高而降低。

图7 12-6 Lennard-Jones势能曲线示意
Fig.7 Schematic diagram of the 12-6 Lennard-Jones potential
在相同的温度下,3层模型中的C值大于单层模型。在页岩比表面积相同的前提下,更大的C值表明在3层模型中的格子划分更加紧密,导致3层模型的排斥作用能(E值)也大于单层模型。3层模型将第2层和第3层也认为吸附层,导致3层模型中页岩表面和被吸附分子平均核间距、吸附相体积均大于单层模型,从而使3层模型中页岩与甲烷分子的吸附作用能(Es的绝对值)和最大吸附相密度ρm均小于单层模型。
3.4 吸附相性质分析
绝对吸附量对于评估页岩气地质储量[50],分析微纳尺度页岩气输送[51-52]和吸附动力学[53]具有重要意义,因此需要准确计算绝对吸附量。通过上述分析,明确了超临界甲烷吸附只能为单层吸附。对于单层吸附,Ono-Kondo格子模型不仅能拟合过剩吸附实验结果,还能直接计算出吸附相密度,根据式(10)可以进一步求得绝对吸附量和吸附相体积。从图8可以看出,绝对吸附量随温度升高而减小。吸附过程中吸附相体积近似保持不变,且在本文的实验压力和温度范围内,吸附相体积几乎不受温度影响。根据分子动力学理论,甲烷与页岩表面的相互作用主要由范德华力控制,该相互作用仅与相对位置有关,与压力和温度无关。对于特定的页岩表面,该相互作用范围应为定值并等于吸附相体积[54]。绝对吸附量随压力单调递增,当压力接近30 MPa时,绝对吸附量尚未接近饱和状态。图8同时表明高压力段绝对吸附量的变化主要受吸附相密度变化影响,因为吸附相体积几乎保持不变。若将吸附相密度假定为定值,由于低压力段过剩吸附量和绝对吸附量近似相等,因此将吸附相密度假定为定值仅能准确预测低压力段的绝对吸附量,但该方法会高估吸附相密度,同时会在整个压力范围内低估吸附相体积,从而导致在高压段对绝对吸附量的低估[12,54]。

图8 不同温度下的甲烷绝对吸附量和吸附相体积
Fig.8 Absolute adsorption of methane and the volume of adsorbed methane under various temperatures
图9显示了吸附相密度随压力增加而单调递增,表明将吸附相密度假设为定值不能准确地表征超临界甲烷在页岩上的吸附。吸附相密度随温度升高而降低,这是因为吸附相“类似液体”的性质,温度越高,密度越低。吸附相密度和气体相密度的差值(ρa-ρb)随着压力增加而先增加后降低。根据方程10可知,在吸附相体积Va近似保持不变的情况下,当吸附相密度ρa增幅超过气体相密度ρb增幅时,过剩吸附量必然出现最大值。

图9 不同温度下吸附相密度和吸附相密度与气体相 密度的差值
Fig.9 Density of adsorbed methane and the difference between the density of adsorbed methane and the density of bulk methane under various temperatures
4 结 论
(1)通过开展重量法高温高压甲烷等温吸附实验,实验结果表明该方法能够同时准确测定气体相甲烷密度和过剩吸附量。在40~100 ℃条件下,实测气体相甲烷密度值平均绝对偏差分别为0.22%,0.18%,1.62%和0.16%。
(2)过剩吸附量随压力先增大后减小,在40~100 ℃条件下,甲烷在四川盆地龙马溪组页岩上最大过剩量为1.724 8~1.232 0 cm3/g(STP)。过剩吸附量随温度升高而逐渐降低,最大过剩吸附量对应的压力随温度升高而升高。
(3)在不对吸附相性质做任何假设的前提下,采用Ono-Kondo模型分别计算了单层和多层吸附,结果表明超临界甲烷在页岩上的吸附只能形成单层吸附,不能形成多层吸附。随着温度升高,页岩与甲烷分子间的吸附作用能,甲烷分子间的排斥能均减弱;温度升高还使得吸附点位减少。
(4)Ono-Kondo模型分析表明:页岩气超临界吸附过程中吸附相体积近似保持不变,且几乎不受温度影响。吸附相密度随压力单调递增,若将吸附相密度假定为定值必然将低估吸附相体积和绝对吸附量。
(5)随着压力增加,吸附相密度ρa与气体相密度ρb的差值先增加后减小,在吸附相体积保持近似不变的情况下,实验所测得的过剩吸附量必然出现最大值。
[1] U.S.Energy Information Administration.Energy Outlook 2020[EB/OL].(2020-01-29)[2021-05-09].https://www.eia.gov/outlooks/aeo/.
[2] U.S.Energy Information Administration.World Shale Resource Assessments[EB/OL].(2015-09-24)[2021-05-09].https://www.eia.gov/analysis/studies/worldshalegas/.
[3] 央广网.2020年我国页岩气产量增长超过3成 成为天然气增产主力军[EB/OL].(2021-02-09)[2021-05-09].2021.http://finance.cnr.cn/txcj/20210209/t20210209_525412040.shtml.
[4] MONTGOMERY S L,JARIVE D M,BOWKER K A,et al.Mississippian Barnett Shale,Fort Worth basin,north-central Texas:Gas-shale play with multi-trillion cubic foot potential[J].American Association of Petroleum Geologists Bulletin,2005,89(2):155-175.
[5] GASPARIK M,BERITIER P,GENSTERBLUM Y,et al.Geological controls on the methane storage capacity in organic-rich shales[J].International Journal of Coal Geology,2014,123(1):34-51.
[6] GASPARIK M,GHANIZADEH A,BERTIER P,et al.High-pressure methane sorption isotherms of black shales from the Netherlands[J].Energy and Fuels,2012,26(8):4995-5004.
[7] SHABANI M,MOALLEMI S A,KROOSS B M,et al.Methane sorption and storage characteristics of organic-rich carbonaceous rocks,Lurestan Province,southwest Iran[J].International Journal of Coal Geology,2018,186(1):51-64.
[8] 周尚文,薛华庆,郭伟,等.基于重量法的页岩气超临界吸附特征实验研究[J].煤炭学报,2016,41(11):2806-2812.
ZHOU Shangwen,XUE Huaqing,GUO Wei,et al.Supercritical isothermal adsorption characteristics of shale gas based on gravimetric method[J].Journal of China Coal Society,2016,41(11):2806-2812.
[9] YANG Feng,NING Zhengfu,ZHANG Rui,et al.Investigations on the methane sorption capacity of marine shales from Sichuan Basin,China[J].International Journal of Coal Geology,2015,146(1):104-117.
[10] XIONG J,LIU X,LIANG L,et al.Adsorption of methane in organic-rich shale nanopores:An experimental and molecular simulation study[J].Fuel,2017,200(15):299-315.
[11] TANG X,RIPEPI N,STADIE N P,et al.A dual-site Langmuir equation for accurate estimation of high pressure deep shale gas resources[J].Fuel,2016,185(1):10-17.
[12] TANG X,RIPEPI N,RIGBY S,et al.New perspectives on supercritical methane adsorption in shales and associated thermodynamics[J].Journal of Industrial and Engineering Chemistry,2019,78(25):186-197.
[13] REXER T F T,BENHAM M J,APLIN A C,et al.Methane adsorption on shale under simulated geological temperature and pressure conditions[J].Energy and Fuels,2013,27(6):3099-3109.
[14] TOPOR T,DERKOWSKI A,ZIEMIANSKI P,et al.The effect of organic matter maturation and porosity evolution on methane storage potential in the Baltic Basin (Poland) shale-gas reservoir[J].International Journal of Coal Geology,2017,180(1):46-56.
[15] TIAN H,LI T,ZHANG T,et al.Characterization of methane adsorption on overmature Lower Silurian-Upper Ordovician shales in Sichuan Basin,southwest China:Experimental results and geological implications[J].International Journal of Coal Geology,2016,156(15):36-49.
[16] PAN L,XIAN X,TIAN H,et al.Geological models of gas in place of the Longmaxi shale in Southeast Chongqing,South China[J].Marine and Petroleum Geology,2016,73:433-444.
[17] LI T,TIAN H,XIAO X,et al.Geochemical characterization and methane adsorption capacity of overmature organic-rich Lower Cambrian shales in northeast Guizhou region,southwest China[J].Marine and Petroleum Geology,2017,86:858-873.
[18] 董银涛,鞠斌山,刘楠楠.页岩甲烷高压等温吸附模型评价与改进[J].煤炭学报,2020,45(9):3208-3218.
DONG Yintao,JU Binshan,LIU Nannan.Evaluation and improvement of high-pressure isothermal adsorption model for methane in shale[J].Journal of China Coal Society,2020,45(9):3208-3218.
[19] 盛茂,李根生,陈立强,等.页岩气超临界吸附机理分析及等温吸附模型的建立[J].煤炭学报,2014,39(S1):179-183.
SHENG Mao,LI Gensheng,CHEN Liqiang,et al.Mechanisms analysis of shale-gas supercritical adsorption and modeling of isorption adsorption[J].Journal of China Coal Society,2014,39(S1):179-183.
[20] SONG X,LÜ X,SHEN Y,et al.A modified supercritical Dubinin-Radushkevich model for the accurate estimation of high pressure methane adsorption on shales[J].International Journal of Coal Geology,2018,193(1):1-15.
[21] MOSHER K,HE J,LIU Y,et al.Molecular simulation of methane adsorption in micro-and mesoporous carbons with applications to coal and gas shale systems[J].International Journal of Coal Geology,2013,109-110(1):36-44.
[22] ZHOU L,ZHOU Y,BAI S,et al.Studies on the transition behavior of physical adsorption from the sub-to the supercritical region:Experiments on silica gel[J].Journal of Colloid and Interface Science,2002,253(1):9-15.
[23] ZHOU L,BAI S,SU W,et al.Comparative study of the excess versus absolute adsorption of CO2 on superactivated carbon for the near-critical region[J].Langmuir,2003,19(7):2683-2690.
[24] LIN K,HUANG X,ZHAO Y.Combining image recognition and simulation to reproduce the adsorption/desorption behaviors of shale gas[J].Energy and Fuels,2020,34(1):258-269.
[25] LI W,PANG X,SNAPE C,et al.Molecular simulation study on methane adsorption capacity and mechanism in clay minerals:Effect of clay type,pressure,and water saturation in shales[J].Energy and Fuels,2019,33(2):765-778.
[26] HILL T L.Adsorption from a one-dimensional lattice gas and the Brunauer-Emmett-Teller equation[J].Proceedings of the National Academy of Sciences of the United States of America,1996,93(25):14328-14332.
[27] ZHOU L,ZHOU Y.Linearization of adsorption isotherms for high-pressure applications[J].Chememical Engineering Science,1998,53(14):2531-2536.
[28] DONOHUE M D,RANOVICH G L.Classification of Gibbs adsorption isotherms[J].Advances in Colloid and Interface Science,1998,76-77:137-152.
[29] GUO L,XIAO L,SHAN X,et al.Modeling adsorption with lattice Boltzmann equation[J].Scientific Report,2016,6:27134-27142.
[30] BI H,JIANG Z,LI J,et al.The Ono-Kondo model and an experimental study on supercritical adsorption of shale gas:A case study of on Longmaxi shale in southeastern Chongqing,China[J].Journal of Natural Gas Science and Engineering,2016,35(A):114-121.
[31] 周尚文,王红岩,薛华庆,等.基于Ono-Kondo格子模型的页岩气超临界吸附机理探讨[J].地球科学,2017,42(8):1421-1430.
ZHOU Shangwen,WANG Hongyan,XUE Huaqing,et al.Discussion on the supercritical adsorption mechanism of shale gas based on ono-kondo lattice model[J].Earth Science,2017,42(8):1421-1430.
[32] ZHOU J,LIU M,XIAN X,et al.Measurements and modelling of CH4 and CO2 adsorption behaviors on shales:Implication for CO2 enhanced shale gas recovery[J].Fuel,2019,251(1):293-306.
[33] ARANOVICH G L,DONOHUED M D.Adsorption of supercritical fluids[J].Journal of Colloid and Interface Science,1996,180(2):537-541.
[34] BENARD P,CHAHINE R.Modeling of high-pressure adsorption isotherms above the critical temperature on microporous adsorbents:Application to methane[J].Langmuir,1997,13(4):808-813.
[35] HWANG J,JOSS L,PINI R.Measuring and modelling supercritical adsorption of CO2 and CH4 on montmorillonite source clay[J].Microporous and Mesoporous Material,2019,273(1):107-121.
[36] GASPARIK M,REXER T F T,APLIN A C,et al.First international inter-laboratory comparison of high-pressure CH4,CO2 and C2H6 sorption isotherms on carbonaceous shales[J].Internatioanl Journal of Coal Geology,2014,132(1):131-146.
[37] LEMMON E W,MCLINDEN M O,FRIEND D G.NIST Chemistry WebBook NIST Standard Reference Database Number 69[DB/OL].(2021-03-06)[2021-05-09].https://webbook.nist.gov/chemistry/.
[38] 俞凌杰,范明,陈红宇,等.富有机质页岩高温高压重量法等温吸附实验[J].石油学报,2015,36(5):557-563.
YU Lingjie,FAN Ming,CHEN Hongyu,et al.Isothermal adsorption experiment of organic-rich shale under high temperature and pressure using gravimetric method[J].Acta Petrolei Sinica,2015,36(5):557-563.
[39] ZHOU S,XUE H,NING Y,et al.Experimental study of supercritical methane adsorption in Longmaxi shale:Insights into the density of adsorbed methane[J].Fuel,2018,211(1):140-148.
[40] 杨峰,宁正福,王庆,等.甲烷在页岩上吸附的热力学.中南大学学报(自然科学版),2014,45(8):2871-2877.
YANG Feng,NING Zhengfu,WANG Qing,et al.Thermodynamic analysis of methane adsorption on gas shale[J].Journal of Central South University (Science and Technology),2014,45(8):2871-2877.
[41] MOSHER K,HE J,LIU Y,et al.Molecular simulation of methane adsorption in micro-and mesoporous carbons with applications to coal and gas shale systems[J].Internatioanl Journal of Coal Geology,2013,109-110:36-44.
[42] MEREY S,SINAYUC C.Analysis of carbon dioxide sequestration in shale gas reservoirs by using experimental adsorption data and adsorption models[J].Journal of Natural Gas Science and Engineering,2016,36(A):1087-1105.
[43] WU C,TUO J,ZHANG L,et al.Pore characteristics differences between clay-rich and clay-poor shales of the Lower Cambrian Niutitang Formation in the Northern Guizhou area,and insights into shale gas storage mechanisms[J].Internatioanl Journal of Coal Geology,2017,178:13-25.
[44] WEI Z,WANG Y,WANG G,et al.Pore characterization of organic-rich Late Permian Da-long Formation shale in the Sichuan Basin,southwestern China[J].Fuel,2018,211:507-516.
[45] QI R,NING Z,WANG Q,et al.Measurements and modeling of high-pressure adsorption of CH4 and CO2 on shales[J].Fuel,2019,242:728-743.
[46] MERKEL A,FINK R,LITTKE R.High pressure methane sorption characteristics of lacustrine shales from the Midland Valley Basin,Scotland[J].Fuel,2016,182:361-372.
[47] SUDIBANDRYO M,MOHAMMAD S A,ROBINSON R L,et al.Ono-Kondo lattice model for high-pressure adsorption:Pure gases[J].Fluid Phase Equilibria,2010,299(2):238-251.
[48] YE Z,CHEN D,PAN Z,et al.An improved Langmuir model for evaluating methane adsorption capacity in shale under various pressures and temperatures[J].Journal of Natural Gas Science and Engineering,2016,31:658-680.
[49] 熊健.页岩对甲烷吸附性能影响因素研究[D].成都:西南石油大学,2015:154-156.
XIONG Jian.Investigation of the influences of the methane adsorption capacity on the shales[D].Chengdu:Southwest Petroleum University,2015:154-156.
[50] FENG G,ZHU Y,CHEN S,et al.Supercritical Methane adsorption on shale over wide pressure and temperature ranges:Implications for gas-in-place estimation[J].Energy and Fuels,2020,34(3):3121-3134.
[51] SONG W,YAO J,MA J,et al.Assessing relative contributions of transport mechanisms and real gas properties to gas flow in nanoscale organic pores in shales by pore network modelling[J].International Journal of Heat and Mass Transfer,2017,113:524-537.
[52] CAO G,LIN M,JIANG W,et al.A 3D coupled model of organic matter and inorganic matrix for calculating the permeability of shale[J].Fuel,2017,204:129-143.
[53] BRANDANI S,MANGANO E,SARKISOV L.Net,excess and absolute adsorption and adsorption of helium[J].Adsorption,2016,22:261-276.
[54] KONG X,FAN H,XIAO D,et al.Improved methane adsorption model in shale by considering variable adsorbed phase density[J].Energy and Fuels,2021,35(3):2064-2074.
