新疆准东、哈密等地区煤炭储量丰富,且此区域煤中硫与灰含量低,是一种优质的动力煤,开发利用潜力巨大[1]。但该煤中钠含量(以灰分计)高于2%,导致其在燃烧利用过程中释放出大量的含钠气态物质,使锅炉受热面等部位出现严重的积灰结渣等问题[2-4],极大限制了相关煤种的推广应用。
煤中钠的存在形式主要分为无机钠和有机钠[5]。在燃烧过程中,煤中钠主要以NaCl和Na原子等途径释放[6-7]。陶玉洁等[8]研究发现,天池煤中的钠在400~600 ℃开始释放,且1 000 ℃前的主要释放形式为气态NaCl,1 000 ℃后为气态NaOH和Na2SO4。刘大海等[9]研究表明,五彩湾煤在815 ℃燃烧时,其中的无机钠大部分以NaCl晶体和水合离子形式被带至煤粒表面释放,大部分有机钠以钠原子的形式释放。
煤燃烧释放出的气态含钠物(NaCl、Na2SO4)易在受热面上冷凝[10],形成具有黏性的内白层,内白层不断捕获烟气中的细微灰颗粒,使灰层厚度增加,并在高温下发生烧结形成致密的烧结层[11]。烧结是指固态颗粒层在低于其熔点的条件下受热互相黏结成团,内部空隙减少,形成致密晶体的过程[10,12]。烧结温度是煤灰开始发生烧结的温度,也是评价煤燃烧过程中结渣倾向的重要指标之一,烧结温度低的灰结渣倾向高,反之亦然[13]。
高钠煤燃烧过程中,含钠物质不仅会进入烟气中导致内白层的形成,也会通过物理、化学方式固定在煤灰中,进而改变煤灰化学组成[11]。煤灰的化学组成影响煤灰的结渣倾向,表现为煤灰碱酸比(B/A)增加,结渣倾向增大。LUAN等[13]研究发现,煤灰B/A低于0.35时,烧结温度随B/A的增大而降低。
学者们大多从煤中钠在燃烧过程中的迁移规律[14-17]及调整高钠煤灰组分[18-21]等对锅炉内结渣情况进行研究。目前含钠蒸气对煤灰烧结的影响研究较少,故笔者以五彩湾煤(WCW)、哈密煤(HM)和平朔煤(PS)为原料,在马弗炉900 ℃下制灰。由于高钠煤燃烧释放的含钠化合物蒸气主要为NaCl[22],因此在不同NaCl蒸气体积分数下对煤灰进行钠捕获实验,并对钠捕获前后的煤灰采用ICP-OES、X-射线衍射和压降法进行表征,分别获得煤灰的钠捕获量、矿物质组成和烧结温度,以此探究NaCl蒸气对煤灰烧结温度的影响,为缓解高钠煤燃烧利用过程中出现的结渣问题提供理论参考。
1 实 验
1.1 实验样品制备
选用新疆准东地区的五彩湾煤(WCW)和哈密地区的哈密煤(HM)2种高钠煤作为研究对象,并以低钠的平朔煤(PS)作为对比煤样。3种煤样的工业分析、元素分析及灰成分分析分别见表1、2。

1.2 实验装置及步骤
1.2.1 原料预处理
依据GB 474—2008中煤样的制备方法对煤样进行预处理,将煤样放入温度为105 ℃的干燥箱中恒温干燥24 h,采用QE-100高速粉碎机将干燥后的煤样破碎,再经研磨筛分出粒径为0.1~1.0 mm的煤样装入密封袋中保存,用于后续制灰实验。
实验用NaCl经研钵研磨并筛分至0.1 mm以下,用于后续的煤灰钠捕获实验。
1.2.2 制灰实验
试验仪器为上海贵尔机械设备有限公司生产的GR/AF12-16马弗炉。称量10 g煤样放入瓷舟中铺平并置于马弗炉中,以10 ℃/min的升温速率从室温升至350 ℃,保温30 min后以5 ℃/min的升温速率升至900 ℃,继续保温60 min,冷却至室温后收集煤灰放入密封袋中用于后续实验与分析测试。
1.2.3 煤灰钠捕获实验
试验仪器为上海昀跃仪器设备有限公司生产的YYSK2-5-12AS型管式炉。图1为实验装置示意。本实验的钠源蒸气原料选用NaCl,其熔点为801 ℃。研究表明,碱金属在750~1 000 ℃的烟气温度下具有强黏性[23],在此区间内受热面极易发生沾污结渣,故NaCl蒸气生成段设计温度为810 ℃[24]。NaCl在810 ℃时的质量变化速率为0.68%/min[25],故设置NaCl载入量为3 g,进入两段炉反应段气体中的NaCl蒸气体积分数按式(1)计算。经计算设置气体体积流量为5、15和25 mL/min时,反应气体中NaCl体积分数分别为76%、51%、38%。

1—钠蒸气生成段;2—钠源载管;3—样品载管;4—反应段
图1 钠捕获实验装置示意
Fig.1 Schematic diagram of sodium capturing experimental device
(1)
式中,H为反应气体中NaCl体积分数,%;Z为NaCl蒸气蒸发量,mg/min;K为空气质量流量,mg/min;S为810 ℃下NaCl的质量变化速率,%/min;N为钠源载入量,g;V为空气体积流量,mL/min;ρ为空气密度,g/mL。
1.3 分析方法
1.3.1 电感耦合等离子原子发射光谱分析
采用上海屹尧仪器科技发展有限公司生产的TOPEX微波消解仪对样品进行消解预处理后,采用美国PerkinElmer公司生产的Optima 8000电感耦合等离子原子发射光谱仪(ICP-OES)进行样品的钠含量测定,实验后每组煤灰均经3次测量后取平均值记为此煤灰的钠含量。
1.3.2 X-射线衍射分析
采用德国BRUKERAXS公司生产的D8 ADVANCE型X射线衍射仪(XRD)分析煤灰样品中的矿物质,扫描方式为固定耦合方式,扫描角度2θ=10°~80°,步距为0.02。
1.3.3 煤灰烧结温度测量(压降法)
采用压降法在图2所示的管式炉中进行煤灰烧结温度的测量,气体流速为15 mL/min。取30 g钠捕获后煤灰放入内径为6 mm的刚玉管中,使用相同内径的玻璃棒从管子两端插入挤压煤灰,得到直径与高度均为6 mm的灰柱,设置升温速率为10 ℃/min。实验过程中实时记录煤灰灰柱温度及其两端压降,压降达到最大值时所对应的温度为煤灰烧结温度。

图2 烧结温度测量装置结构示意
Fig.2 Schematic diagram of sintering temperature measuring device
2 实验结果与分析
2.1 不同煤灰的钠捕获性能
由ICP-OES分析结果可知,WCW、HM和PS煤灰的钠初始质量分数分别为3.49、3.73 和0.19 μg/g。图3为不同体积分数NaCl蒸气下煤灰的钠捕获量。与捕获前相比,WCW煤灰在38%、51%、76% NaCl蒸气体积分数下的钠质量分数分别增加了0.01、0.03、0.07 μg/g,HM煤灰分别增加了0.05、0.06、0.08 μg/g,而PS煤灰分别增加了0.10、0.12、0.13 μg/g。3种煤样在不同体积分数NaCl蒸气下的钠捕获量均依次为PS>HM>WCW。

图3 不同NaCl蒸气体积分数下煤灰的钠捕获量
Fig.3 Sodium capturing amount of the coal ash under the different NaCl vapor concentration
由表2可知,3种煤样灰成分中的硅、铝含量由高到低依次为PS>HM>WCW,同时3种煤灰中钙含量由低到高为PS
表2 煤样的灰成分分析
Table 2 Ash analyses of the coal samples %
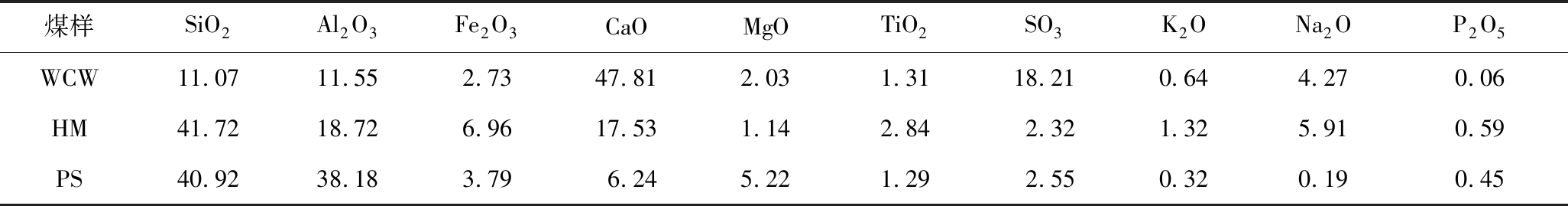
煤样SiO2Al2O3Fe2O3CaOMgOTiO2SO3K2ONa2OP2O5WCW11.0711.552.7347.812.031.3118.210.644.270.06HM41.7218.726.9617.531.142.842.321.325.910.59PS40.9238.183.796.245.221.292.550.320.190.45
2NaCl+H2O![]() Na2O+2HCl
Na2O+2HCl
(2)
Na2O+Al2O3+6SiO2![]() Na2Al2Si6O16
Na2Al2Si6O16
(3)
Na2O+2SiO2+Al2O3![]() 2NaAlSiO4
2NaAlSiO4
(4)
2CaO+Al2O3+SiO2![]() Ca2Al2SiO7
Ca2Al2SiO7
(5)
随NaCl蒸气体积分数升高,3种煤灰钠捕获量增幅由大到小为WCW>HM>PS,表明NaCl蒸气体积分数的改变对WCW煤灰钠捕获量的影响比HM和PS更大。
2.2 钠捕获前后煤灰中矿物质的衍变
图4为不同NaCl蒸气体积分数下,钠捕获前后的XRD图谱。由图4可知,与钠捕获前WCW煤灰相比,钠捕获后WCW煤灰的XRD图谱中SiO2、Ca4Si3O9(OH)2、Ca3Si3O9、Na6CaAl6Si6O24(SO4)2和Fe2O3的衍射峰消失,NaAlSiO4、Ca3SiO5、Na3K(AlSiO4)4、Na2CaSiO4和Na2Al2Si6O16的衍射峰出现,表明在钠捕获过程中,煤灰中的硅铝矿物质(如SiO2、Ca4Si3O9(OH)2、Ca3Si3O9和Na6CaAl6Si6O24(SO4))2以及煤灰中含钾物质通过反应(3)、(4)、(6)、(7)生成NaAlSiO4、Na2Al2Si6O16、Na2CaSiO4和Na3K(AlSiO4)4。碱金属离子(Na+、K+等)能使硅酸盐熔体的桥氧键断裂,进而使煤灰中矿物质晶格重组相变为骨架更小的低熔点矿物质[29-30]。当煤灰中SiO2晶格结构中半数的[SiO4]-被替换成[AlO4]5-时,晶格结构发生扭曲,K+和Na+补偿替换后的多余负电荷,生成NaAlSiO4和Na3K(AlSiO4)4[30]。此外,Fe2O3与NaCl可能发生反应生成FeCl3(反应(8)),而FeCl3随烟气挥发[31],因此,XRD图谱中Fe2O3衍射峰消失,也未发现FeCl3的衍射峰。

a—CaSO4;b—SiO2;c—Ca4Si3O9(OH)2;d—Ca12Al14O33;e—NaAlSiO4;f—Ca3Si3O9;g—Ca3SiO5;h—Na3K(AlSiO4)4;i—Na2CaSiO4;j—Na6CaAl6Si6O24(SO4)2;k—Na2Al2Si6O16;s—Fe2O3
图4 不同NaCl蒸气体积分数下WCW煤灰XRD图谱
Fig.4 XRD patterns of Wucaiwan coal ash under the different NaCl vapor concentration
Na2O+CaO+SiO2![]() Na2CaSiO4
Na2CaSiO4
(6)
3Na2O+K2O+2Al2O3+4Al2O3+8SiO2![]() 2Na3K(AlSiO4)4
2Na3K(AlSiO4)4
(7)
Fe2O3+6NaCl![]() 2FeCl3+3Na2O
2FeCl3+3Na2O
(8)
随着NaCl蒸气体积分数上升,NaAlSiO4、Na2Al2Si6O16、Na2CaSiO4、Na3K(AlSiO4)4、Ca3SiO5和Ca12Al14O33的衍射峰强度增强,CaSO4的衍射峰强度则不断减弱,且Na3K(AlSiO4)4衍射峰增强幅度大于NaAlSiO4衍射峰增强幅度。当NaCl蒸气体积分数升高时,反应(3)、(4)、(6)、(7)、(9)、(10)[20]增强,消耗的含钙物质增多,导致反应(11)不断增强,CaSO4的衍射峰强度不断降低,同时反应(11)生成的CaO和硅铝物质与NaCl发生共熔现象[32],所以NaAlSiO4、Na2CaSiO4、Ca3SiO5、Na2Al2Si6O16及Na3K(AlSiO4)4的衍射峰强度增强[33]。Na3K(AlSiO4)4衍射峰的增强幅度比NaAlSiO4更大的原因,可能是NaCl蒸气体积分数增大的同时,Na+浓度也不断增大,同时煤灰中含钾物质的反应活性更强,因而Na3K(AlSiO4)4先于NaAlSiO4生成。此现象表明随着NaCl蒸气体积分数增大,WCW煤灰中硅铝物质固钠反应增强,从而使得煤灰固钠能力提高。
3CaO+SiO2![]() Ca3SiO5
Ca3SiO5
(9)
12CaO+7Al2O3![]() Ca12Al14O33
Ca12Al14O33
(10)
CaSO4![]() CaO+SO3
CaO+SO3
(11)
CaO+Al2O3+2SiO2![]() CaAl2Si2O8
CaAl2Si2O8
(12)
6NaAlSiO4+K2O+Al2O3+2SiO2![]() 2Na3K(AlSiO4)4
2Na3K(AlSiO4)4
(13)
由图5可知,HM煤灰的XRD图谱中主要矿物质的衍射峰为CaSO4、SiO2、NaAlSiO4、Ca2Al2SiO7、CaAl2Si2O8及Ca3Al6Si2O16。与钠捕获前相比,钠捕获后HM煤灰的XRD图谱中CaSO4、Ca2Al2SiO7衍射峰消失,而Na3K(AlSiO4)4和CaSiO3衍射峰出现,CaAl2Si2O8衍射峰增强,表明HM煤灰在Na捕获过程中发生反应生成了Na3K(AlSiO4)4和CaAl2Si2O8(反应(7)、(12))。此时,除煤灰中的CaSO4按反应(11)分解外,还发生了Ca2Al2SiO7向CaAl2Si2O8的转化反应[34-35]。

a—CaSO4;b—SiO2;e—NaAlSiO4;m—Ca2Al2SiO7;n—CaAl2Si2O8;p—CaSiO3;h—Na3K(AlSiO4)4;q—Ca3Al6Si2O16
图5 不同NaCl蒸气体积分数下HM煤灰XRD图谱
Fig.5 XRD patterns of Hami coal ash under the different NaCl vapor concentration
随NaCl蒸气体积分数增大,HM煤灰XRD图谱中的SiO2、NaAlSiO4和Ca3Al6Si2O16衍射峰强度减弱,Na3K(AlSiO4)4和CaSiO3的衍射峰强度不断增强,表明NaCl蒸气体积分数增大促进了Ca3Al6Si2O16的分解,NaAlSiO4可能与SiO2及煤灰中的含钾物质反应生成Na3K(AlSiO4)4(式(13))。另外,由于HM煤灰中钾含量高于WCW煤灰,故在NaCl蒸气体积分数增大时,Na+与煤灰的含钾物质优先生成Na3K(AlSiO4)4,进而导致NaAlSiO4的生成量减少。CaSiO3的衍射峰强度不断增强表明,HM煤灰中的CaSO4等含钙物质也与SiO2等硅铝物质反应生成CaSiO3等物质,从而抑制了硅铝对钠的固定。WCW煤灰中生成的含钠物质为NaAlSiO4、Na3K(AlSiO4)4、Na2CaSiO4和Na2Al2Si6O16而HM煤灰中生成的含钠物质为Na3K(AlSiO4)4,WCW煤灰生成固钠能力较强的Na2Al2Si6O16,而Na3K(AlSiO4)4的固钠能力较弱,因此WCW煤灰的钠捕获增量比HM煤灰有所增加。
如图6所示,PS煤灰XRD图谱中的主要矿物质衍射峰为CaSO4和SiO2。经钠捕获,PS煤灰的XRD图谱均出现了NaAlSiO4和Na2Al2Si6O16的衍射峰,而CaSO4和SiO2的衍射峰强度减弱,表明此时煤灰中的硅铝物质与NaCl蒸气发生了反应(3)、(4)并将其固定。随NaCl蒸气体积分数增大,NaAlSiO4和Na2Al2Si6O16的衍射峰强度不断增强,而CaSO4和SiO2的衍射峰强度不断减弱,可能是由于PS煤灰在钠捕获的过程中主要发生反应(3)、(4)、(11),导致不断消耗CaSO4和SiO2,同时生成NaAlSiO4和Na2Al2Si6O16,且此反应随NaCl蒸气体积分数的上升不断。PS煤灰中生成的含钠物质为NaAlSiO4和Na2Al2Si6O16。结合表2,PS煤灰中钾含量最低,仅为0.32%,因此,PS煤灰在钠捕获过程中不生成或生成较少的Na3K(AlSiO4)4,且生成固钠能力较强的Na2Al2Si6O16,而不是固钠能力较弱的Na3K(AlSiO4)4,从而导致PS煤灰的钠捕获能力在3种煤灰中最高。

a—CaSO4;b—SiO2;e—NaAlSiO4;k—Na2Al2Si6O16
图6 不同NaCl蒸气体积分数下PS煤灰XRD图谱
Fig.6 XRD patterns of Pingshuo coal ash under the different NaCl vapor concentration
2.3 煤灰固钠后烧结温度的衍变
图7为不同NaCl蒸气体积分数下钠捕获前后煤灰的烧结温度。初始煤灰的烧结温度由高到低依次为PS>HM>WCW。煤灰的烧结温度与其钠含量关系密切,理论上煤灰中钠含量越高,其烧结温度越低[10]。初始煤灰中钠含量由小到大为PS

图7 不同NaCl蒸气体积分数下钠捕获前后煤灰烧结温度
Fig.7 Sintering temperature of the coal ash before and after sodium capture under the different NaCl vapor concentration
与初始煤灰烧结温度相比,体积分数为38%的NaCl蒸气下,钠捕获后煤灰烧结温度的降幅由大到小依次为PS>HM>WCW,其中PS煤灰烧结温度降低了97.7 ℃。由2.1节可知,钠捕获量从大到小为PS>HM>WCW,因此灰烧结温度的降低幅度与钠捕获量的大小直接相关,钠捕获量反映了煤灰中含钠矿物质含量,而矿物质相变是引起熔融温度变化的重要原因[36]。由图7可知,随着NaCl蒸气体积分数的增加,3种煤灰的烧结温度持续下降。相比38% NaCl蒸气下的煤灰,76% NaCl蒸气下的WCW、HM、PS三种煤灰的烧结温度降低幅度分别为55.2、37.6、22.2 ℃。WCW煤灰烧结温度受NaCl蒸气含量的影响最大,PS煤灰烧结温度所受影响最小。
由2.2节可知,PS煤灰钠捕获后生成的含钠物质为Na2Al2Si6O16和NaAlSiO4,HM煤灰中生成的含钠物质为Na3K(AlSiO4)4,WCW煤灰中生成的含钠物质有Na3K(AlSiO4)4、Na2Al2Si6O16、NaAlSiO4和Na2CaSiO4。随着NaCl蒸气体积分数升高,助熔离子Na+浓度随之升高,继续与煤灰中的矿物质体系反应,并通过活性较高的氧原子位进入矿物质晶格中,促使煤灰中矿物质继续发生相变生成低熔点化合物[36]。Na3K(AlSiO4)4是一种架状硅酸盐类助熔矿物[28],易导致煤灰中共熔反应的发生,尽管其熔点高于Na2Al2Si6O16,但更易与煤灰中其他矿物质形成低温共熔体,比Na2Al2Si6O16更易降低煤灰烧结温度[19]。因此,PS煤灰烧结温度受NaCl蒸气体积分数的变化影响最小,而固钠后WCW煤灰中的低熔点矿物质更多,烧结温度受NaCl蒸气体积分数的影响最大。此外,在900 ℃的钠捕获过程中,WCW煤和HM煤灰已发生烧结,烧结后的煤灰致密,而PS煤灰未发生明显烧结,其结构疏松,孔隙结构较多,因此未发生明显烧结的PS煤灰可能吸附更多的NaCl蒸气,易与NaCl蒸气反应完全,所以PS煤灰的烧结温度受NaCl蒸气体积分数的变化影响更小。
3 结 论
(1)随NaCl蒸气体积分数升高,3种煤灰的钠捕获量增大。煤灰中硅铝物质能提高煤灰的钠捕获能力,钙则会与煤灰中硅铝物质反应导致其Na捕获能力下降。
(2)煤灰烧结温度主要与煤灰中低熔点含钠化合物(Na3K(AlSiO4)4、NaAlSiO4、Na2CaSiO4和Na2Al2Si6O16)有关。
(3)煤灰的烧结温度降幅与其钠捕获量呈正相关。随NaCl蒸气体积分数升高,煤灰与NaCl蒸气的化学反应能力增强,Na+不断进入煤灰矿物质的晶格结构,Na3K(AlSiO4)4、Na2Al2Si6O16、NaAlSiO4等低熔点含钠化合物增多而使烧结温度降低。
(4)900 ℃的钠捕获过程中,PS煤灰未发生烧结,其烧结温度受NaCl蒸气体积分数的影响较小;而WCW与HM煤灰则发生烧结,其烧结温度受NaCl蒸气体积分数的影响较大。
[1] 张守玉,江锋浩,黄小河,等.五彩湾煤半焦水热脱钠提质及其燃烧特性的演变[J].煤炭学报,2018,43(1):250-256.
ZHANG Shouyu,JIANG Fenghao,HUANG Xiaohe,et al.Investigation on the Na removal and combustion characteristics of the hydrothermal treated Wucaiwan coal char[J].Journal of China Coal Society,2018,43(1):250-256.
[2] 李腾,赵京,魏小林.高碱煤热利用过程中碱金属释放特性的研究进展[J].华中科技大学学报(自然科学版),2023,51(1):92-100.
LI Teng,ZHAO Jing,WEI Xiaolin.Research progress of alkali metals release characteristics during thermal utilization of high-alkali coals[J].Journal of Huazhong University of Science and Technology (Natural Science Edition),2023,51(1):92-100.
[3] 刘大海,张守玉,涂圣康,等.五彩湾煤中钠在热解过程中的形态变迁[J].燃料化学学报,2014,42(10):1190-1196.
LIU Dahai,ZHANG Shouyu,TU Shengkang,et al.Transformation of sodium during Wucaiwan coal pyrolysis[J].Journal of Fuel Chemistry and Technology,2014,42(10):1190-1196.
[4] 马达夫,张守玉,何翔,等.燃准东煤锅炉受热面沾污的燃烧优化试验研究[J/OL].煤炭科学技术:1-9[2022-12-25].DOI:10.13199/j.cnki.cst.2022-0941.
MA Dafu,ZHANG Shouyu,HE Xiang,et al.Experimental study on combustion optimization to alleviate fouling on heating surfaces of a pulverized coal-fired boiler[J/OL].Coal Science and Technology:1-9[2022-12-25].DOI:10.13199/j.cnki.cst.2022-0941.
[5] 刘大海,张守玉,陈川,等.新疆高钠煤脱钠提质过程中钠存在形式[J].煤炭学报,2014,39(12):2519-2524.
LIU Dahai,ZHANG Shouyu,CHEN Chuan,et al.Existence form of sodium in the high sodium coals from Xinjiang during its sodium removal process[J].Journal of China Coal Society,2014,39(12):2519-2524.
[6] RAASK E.Mineral impurities in coal combustion[M].Washington:
Hemisphere Publishing Corporation,1985:97-101.
[7] XU L,LIU H,ZHAO D,et al.Transformation mechanism of sodium during pyrolysis of Zhundong coal[J].Fuel,2018,233:29-36.
[8] 陶玉洁,张彦威,周俊虎,等.准东煤在燃烧过程中的矿物演变过程及Na、Ca释放规律[J].中国电机工程学报,2015,35(5):1169-1175.
TAO Yujie,ZHANG Yanwei,ZHOU Junhu,et al.Mineral conversion regularity and release behavior of Na,Ca during Zhundong coal’s combustion[J].Proceedings of the CSEE,2015,35(5):1169-1175.
[9] 刘大海,张守玉,涂圣康,等.五彩湾煤中钠在燃烧过程中的迁移释放规律[J].化工进展,2015,34(3):705-709.
LIU Dahai,ZHANG Shouyu,TU Shengkang,et al.Transformation and release of sodium in Wucaiwan coal during combustion[J].Chemical Industry and Engineering Progress,2015,34(3):705-709.
[10] 黄东东,张守玉,常明,等.高钠煤灰烧结特性研究进展[J].洁净煤技术,2021,27(1):83-94.
HUANG Dongdong,ZHANG Shouyu,CHANG Ming,et al.Research progress on sintering characteristics of high sodium coal ash[J].Clean Coal Technology,2021,27(1):83-94.
[11] 江锋浩,张守玉,黄小河,等.高碱煤燃烧过程中结渣机理研究进展[J].煤炭转化,2018,41(2):1-8.
JIANG Fenghao,ZHANG Shouyu,HUANG Xiaohe,et al.Research progress on slagging mechanism during high alkali coal combustion process[J].Coal Conversion,2018,41(2):1-8.
[12] NIU Y,TAN H,HUI S.Ash-related issues during biomass combustion:Alkali-induced slagging,silicate melt-induced slagging (ash fusion),agglomeration,corrosion,ash utilization,and related countermeasures[J].Progress in Energy and Combustion Science,2016,52:51-61.
[13] LUAN C,YOU C,ZHANG D.Composition and sintering characteristics of ashes from co-firing of coal and biomass in a laboratory-scale drop tube furnace[J].Energy,2014,69:562-570.
[14] 张守玉,陈川,施大钟,等.高钠煤燃烧利用现状[J].中国电机工程学报,2013,33(5):1-12,17.
ZHANG Shouyu,CHEN Chuan,SHI Dazhong,et al.Situation of combustion utilization of high sodium coal[J].Proceedings of the CSEE,2013,33(5):1-12,17.
[15] 祁超.准东煤燃烧过程中钠的分布规律[D].北京:华北电力大学,2018.
QI Chao.Sodium distribution when burning Zhundong coal[D].Beijing:North China Electric Power University ,2018.
[16] 陈川,张守玉,刘大海,等.新疆高钠煤中钠的赋存形态及其对燃烧过程的影响[J].燃料化学学报,2013,41(7):832-838.
CHEN Chuan,ZHANG Shouyu,LIU Dahai,et al.Existence form of sodium in high sodium coals from Xinjiang and its effect on combustion process[J].Journal of Fuel Chemistry and Technology,2013,41(7):832-838.
[17] 史航,吴玉新,吕俊复,等.循环灰吸附碱金属机理研究[J].煤炭学报,2017,42(4):1063-1068.
SHI Hang,WU Yuxin,LÜ Junfu,et al.Mechanism study of adsorption of alkali with bed material[J].Journal of China Coal Society,2017,42(4):1063-1068.
[18] 高满达,孙保民,苏逸峰.准东煤掺烧NH4H2PO4对灰分变化和灰熔融特性的影响[J].中国电机工程学报,2018,38(20):6012-6020.
GAO Manda,SUN Baomin,SU Yifeng.Influence of NH4H2PO4 on ash composition and ash-forming characteristics during combustion of Zhundong coal[J].Proceedings of the CSEE,2018,38(20):6012-6020.
[19] 王东旭.碱土金属氧化物对准东煤灰熔融特性的影响研究[D].北京:华北电力大学,2018.
WANG Dongxu.Effect of alkaline earth metal oxides on the ash fusibility of Zhundong coal[D].Beijing:Electric Power University,2018.
[20] 高满达.准东煤燃烧中钠行为及富磷添加剂对燃烧与灰熔融特性影响[D].北京:华北电力大学,2019.
GAO Manda.Sodium behavior in Zhundong coal Combustion and effect of phosphorus-rich additives on combustion and ash fusion characteristics[D].Beijing:North China Electric Power University,2019.
[21] 金涛,张守玉,施登宇,等.高钠煤调湿后微波脱钠处理实验研究[J].热能动力工程,2016,31(4):95-99,148-149.
JIN Tao,ZHANG Shouyu,SHI Dengyu,et al.Experimental study on the microwave treatment to remove sodium from high sodium coal after humidifying[J].Journal of Engineering for Thermal Energy and Power,2016,31(4):95-99,148-149.
[22] 黄东东,张守玉,江锋浩,等.固钠剂对NaCl蒸气的钠捕获性能研究[J].中国电机工程学报,2021,41(21):7403-7410.
HUANG Dongdong,ZHANG Shouyu,JIANG Fenghao,et al.Study on the sodium capture performance of sodium adsorbent for NaCl vapor[J].Proceedings of the CSEE,2021,41(21):7403-7410.
[23] JIN J,WANG Y Z,LIU D Y,et al.Understanding ash deposition for Zhundong coal combustion in 330 MW utility boiler:Focusing on surface tem-perature effects[J].Fuel,2018,216:697-706.
[24] JIANG F,ZHANG S,HUANG X,et al.Sodium vapor capture by
coal ash during sodium-rich coal combustion[J].Applied Thermal Engineering,2019,161:114-127.
[25] BROSTRÖM M,ENESTAM S,BACKMAN R,et al.Condensation in the KCl-NaCl system[J].Fuel Processing Technology,2013,105:142-148.
[26] 郎森,张守玉,常明,等.逐级萃取对高钠煤灰钠捕获性能的影响[J].煤炭学报,2022,47(12):4517-4525.
LANG Sen,ZHANG Shouyu,CHANG Ming,et al.Effect of sequential extraction on sodium capture capabilitu of sodium-rich coal ash[J].Journal of China Coal Society,2022,47(12):4517-4525.
[27] 陈午凤,王长安,魏邓昶泓,等.准东煤O2/CO2燃烧过程中硅铝矿物添加剂对钠/钙/铁释放与迁移的影响[J].新能源进展,2017,5(1):16-22.
CHEN Wufeng,WANG Chang’an,WEI Dengchanghong,et al.Influences of salic mineral additives on migration of Na/Ca/Fe during O2/CO2 combustion of Zhundong coals[J].Advances in New and Renewable Energy,2017,5(1):16-22.
[28] 刘炎泉,程乐鸣,季杰强,等.添加剂对高碱煤钠迁移和灰分烧结温度的影响[J].燃料化学学报,2018,46(11):1298-1304.
LIU Yanquan,CHENG Leming,JI Jieqiang,et al.Influence of additives on sodium release and ash sintering temperature of a high-alkali coal[J].Journal of Fuel Chemistry and Technology,2018,46(11):1298-1304.
[29] 陈玉爽,张忠孝,乌晓江,等.配煤对煤灰熔融特性影响的实验与量化研究[J].燃料化学学报,2009,37(5):521-526.
CHEN Yushuang,ZHANG Zhongxiao,WU Xiaojiang,et al.Quantum chemistry calculation and experimental study on coal ash fusion characteristics of blend coal[J].Journal of Fuel Chemistry and Technology,2009,37(5):521-526.
[30] 刘欢欢,杜梅芳,王要克,等.碱金属对煤灰熔融特性的影响机理[J].材料科学与工程学报,2018,36(6):985-992.
LIU Huanhuan,DU Meifang,WANG Yaoke,et al.Characteristics and mechanism on coal ash fusion[J].Journal of Materials Science and Engineering,2018,36(6):985-992.
[31] 马达夫,何翔,周文台,等.新疆高碱煤沾污特性的分析[J].发电设备,2016,30(1):7-10.
MA Dafu,HE Xiang,ZHOU Wentai,et al.Analysis on fouling characteristics of Xinjiang high-alkali coal[J].Power Equipment,2016,30(1):7-10.
[32] 江锋浩.高钠煤燃烧过程中钠捕获研究[D].上海:上海理工大学,2019.
JIANG Fenghao.Study on sodium captured during sodium-rich coal combustion[D].Shanghai:University of Shanghai for Science and Technology,2019.
[33] 黄小河,张守玉,杨靖宁,等.准东煤高温燃烧过程中含钙矿物质的转化规律[J].化工学报,2017,68(10):3906-3911.
HUANG Xiaohe,ZHANG Shouyu,YANG Jingning,et al.Calcium transformation during Zhundong coal combustion process[J].CIESC Journal,2017,68(10):3906-3911.
[34] 田思达,李乐天,吴玉新,等.氧化硅添加剂对准东煤高钙飞灰物相热转化作用的差示扫描量热分析[J].中国电机工程学报,2020,40(15):4919-4927.
TIAN Sida,LI Letian,WU Yuxin,et al.Investigation of the role of silica additive in thermal transformation of Zhundong coal high-calcium fly ash by DSC[J].Proceedings of the CSEE,2020,40(15):4919-4927.
[35] 常可可.循环流化床锅炉粉煤灰理化性质研究[D].太原:山西大学,2017.
CHANG Keke.Characteristics of fly ash and slag in circulating fluidized bed boiler[D].Taiyuan:Shanxi University,2017.
[36] 李洁.用量子理论方法对煤灰微观结构特性的研究[D].上海:上海理工大学,2008.
LI Jie.Study on the microstructure of coal ash by quantum theory[D].Shanghai:University of Shanghai for Science and Technology,2008.
